Mast Cell Activation and KSHV Infection in Kaposi Sarcoma
- PMID: 30084838
- PMCID: PMC6191350
- DOI: 10.1158/1078-0432.CCR-18-0873
Mast Cell Activation and KSHV Infection in Kaposi Sarcoma
Abstract
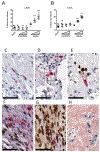
Purpose: Kaposi sarcoma (KS) is a vascular tumor initiated by infection of endothelial cells (ECs) with KS-associated herpesvirus (KSHV). KS is dependent on sustained proinflammatory signals provided by intralesional leukocytes and continued infection of new ECs. However, the sources of these cytokines and infectious virus within lesions are not fully understood. Here, mast cells (MCs) are identified as proinflammatory cells within KS lesions that are permissive for, and activated by, infection with KSHV.Experimental Design: Three validated MC lines were used to assess permissivity of MCs to infection with KSHV and to evaluate MCs activation following infection. Biopsies from 31 AIDS-KS cases and 11 AIDS controls were evaluated by IHC for the presence of MCs in KS lesions and assessment of MC activation state and infection with KSHV. Plasma samples from 26 AIDS-KS, 13 classic KS, and 13 healthy adults were evaluated for levels of MC granule contents tryptase and histamine.Results: In culture, MCs supported latent and lytic KSHV infection, and infection-induced MC degranulation. Within KS lesions, MCs were closely associated with spindle cells. Furthermore, MC activation was extensive within patients with KS, reflected by elevated circulating levels of tryptase and a histamine metabolite. One patient with clinical signs of extensive MC activation was treated with antagonists of MC proinflammatory mediators, which resulted in a rapid and durable regression of AIDS-KS lesions.Conclusions: Using complimentary in vitro and in vivo studies we identify MCs as a potential long-lived reservoir for KSHV and a source of proinflammatory mediators within the KS lesional microenvironment. In addition, we identify MC antagonists as a promising novel therapeutic approach for KS. Clin Cancer Res; 24(20); 5085-97. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures




References
-
- Hagiwara K, Khaskhely NM, Uezato H, Nonaka S. Mast cell “densities” in vascular proliferations: a preliminary study of pyogenic granuloma, portwine stain, cavernous hemangioma, cherry angioma, Kaposi’s sarcoma, and malignant hemangioendothelioma. Journal of Dermatology 1999;26(9):577–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

