Induction of a transmissible tau pathology by traumatic brain injury
- PMID: 30084913
- PMCID: PMC6113646
- DOI: 10.1093/brain/awy193
Induction of a transmissible tau pathology by traumatic brain injury
Abstract
Traumatic brain injury is a risk factor for subsequent neurodegenerative disease, including chronic traumatic encephalopathy, a tauopathy mostly associated with repetitive concussion and blast, but not well recognized as a consequence of severe traumatic brain injury. Here we show that a single severe brain trauma is associated with the emergence of widespread hyperphosphorylated tau pathology in a proportion of humans surviving late after injury. In parallel experimental studies, in a model of severe traumatic brain injury in wild-type mice, we found progressive and widespread tau pathology, replicating the findings in humans. Brain homogenates from these mice, when inoculated into the hippocampus and overlying cerebral cortex of naïve mice, induced widespread tau pathology, synaptic loss, and persistent memory deficits. These data provide evidence that experimental brain trauma induces a self-propagating tau pathology, which can be transmitted between mice, and call for future studies aimed at investigating the potential transmissibility of trauma associated tau pathology in humans.
Figures
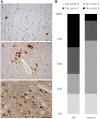

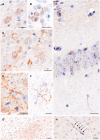
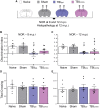
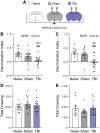
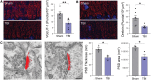
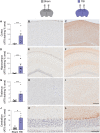
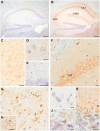
Comment in
-
Traumatic brain injury induces transmissible tau pathology.Nat Rev Neurol. 2018 Oct;14(10):570-571. doi: 10.1038/s41582-018-0062-3. Nat Rev Neurol. 2018. PMID: 30104746 No abstract available.
-
TauBI or not TauBI: what was the question?Brain. 2018 Sep 1;141(9):2536-2539. doi: 10.1093/brain/awy225. Brain. 2018. PMID: 30169590 Free PMC article.
References
-
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 1995; 16: 271–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

