Bacterial secretion chaperones: the mycobacterial type VII case
- PMID: 30085058
- PMCID: PMC6109436
- DOI: 10.1093/femsle/fny197
Bacterial secretion chaperones: the mycobacterial type VII case
Abstract
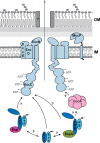
Chaperones are central players in maintaining the proteostasis in all living cells. Besides highly conserved generic chaperones that assist protein folding and assembly in the cytosol, additional more specific chaperones have evolved to ensure the successful trafficking of proteins with extra-cytoplasmic locations. Associated with the distinctive secretion systems present in bacteria, different dedicated chaperones have been described that not only keep secretory proteins in a translocation competent state, but often are also involved in substrate targeting to the specific translocation channel. Recently, a new class of such chaperones has been identified that are involved in the specific recognition of substrates transported via the type VII secretion pathway in mycobacteria. In this minireview, we provide an overview of the different bacterial chaperones with a focus on their roles in protein secretion and will discuss in detail the roles of mycobacterial type VII secretion chaperones in substrate recognition and targeting.
Figures

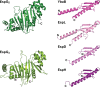
References
-
- Abdallah AM, Verboom T, Hannes F et al. . A specific secretion system mediates PPE41 transport in pathogenic mycobacteria. Mol Microbiol 2006;62:667–79. - PubMed
-
- Abdallah AM, Verboom T, Weerdenburg EM et al. . PPE and PE_PGRS proteins of Mycobacterium marinum are transported via the type VII secretion system ESX-5. Mol Microbiol 2009;73:329–40. - PubMed
-
- Ates LS, Dippenaar A, Ummels R et al. . Mutations in ppe38 block PE_PGRS secretion and increase virulence of Mycobacterium tuberculosis. Nat Microbiol 2018;3:181–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

