The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience
- PMID: 30085354
- PMCID: PMC6367070
- DOI: 10.1111/nyas.13948
The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience
Abstract
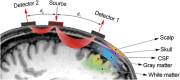
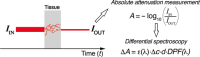
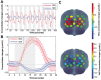
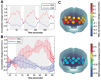
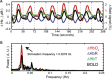
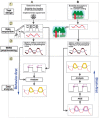
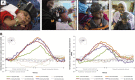
The past few decades have seen a rapid increase in the use of functional near-infrared spectroscopy (fNIRS) in cognitive neuroscience. This fast growth is due to the several advances that fNIRS offers over the other neuroimaging modalities such as functional magnetic resonance imaging and electroencephalography/magnetoencephalography. In particular, fNIRS is harmless, tolerant to bodily movements, and highly portable, being suitable for all possible participant populations, from newborns to the elderly and experimental settings, both inside and outside the laboratory. In this review we aim to provide a comprehensive and state-of-the-art review of fNIRS basics, technical developments, and applications. In particular, we discuss some of the open challenges and the potential of fNIRS for cognitive neuroscience research, with a particular focus on neuroimaging in naturalistic environments and social cognitive neuroscience.
Keywords: basics of fNIRS; cognitive neuroscience; ecological; fNIRS; social neuroscience.
© 2018 The Authors. Annals of the New York Academy of Sciences published by Wiley Periodicals, Inc. on behalf of New York Academy of Sciences.
Figures

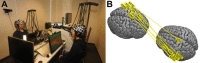
References
-
- Lloyd‐Fox, S. , Blasi A. & Elwell C.E.. 2010. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 34: 269–284. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

