Heparan sulfate is a plasma biomarker of acute cellular allograft rejection
- PMID: 30086133
- PMCID: PMC6080752
- DOI: 10.1371/journal.pone.0200877
Heparan sulfate is a plasma biomarker of acute cellular allograft rejection
Abstract
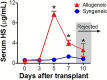
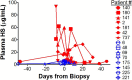
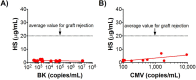
Despite advances in management of immunosuppression, graft rejection remains a significant clinical problem in solid organ transplantation. Non-invasive biomarkers of graft rejection can facilitate earlier diagnosis and treatment of acute rejection. The purpose of this study was to investigate the potential role of heparan sulfate as a novel biomarker for acute cellular rejection. Heparan sulfate is released from the extracellular matrix during T-cell infiltration of graft tissue via the action of the enzyme heparanase. In a murine heart transplant model, serum heparan sulfate is significantly elevated during rejection of cardiac allografts. Moreover, expression of the enzyme heparanase is significantly increased in activated T-cells. In human studies, plasma heparan sulfate is significantly elevated in kidney transplant recipients with biopsy-proven acute cellular rejection compared to healthy controls, recipients with stable graft function, and recipients without acute cellular rejection on biopsy. Taken together, these findings support further investigation of heparan sulfate as a novel biomarker of acute cellular rejection in solid organ transplantation.
Conflict of interest statement
This study received support from the Biomarker Factory, jointly founded and co-owned by Duke Medical Strategies, an affiliate of Duke University, and Laboratory Corporation of America Holdings (LabCorp). There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures






References
-
- Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(3):450–62. 10.1111/j.1600-6143.2010.03283.x . - DOI - PubMed
-
- Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(6):1226–35. 10.1111/j.1600-6143.2011.03539.x . - DOI - PubMed
-
- Tumova S, Woods A, Couchman JR. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. The international journal of biochemistry & cell biology. 2000;32(3):269–88. Epub 2000/03/15. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

