Disruption of C1galt1 Gene Promotes Development and Metastasis of Pancreatic Adenocarcinomas in Mice
- PMID: 30086262
- PMCID: PMC6219903
- DOI: 10.1053/j.gastro.2018.08.007
Disruption of C1galt1 Gene Promotes Development and Metastasis of Pancreatic Adenocarcinomas in Mice
Abstract
Background & aims: Pancreatic ductal adenocarcinomas (PDACs) produce higher levels of truncated O-glycan structures (such as Tn and sTn) than normal pancreata. Dysregulated activity of core 1 synthase glycoprotein-N-acetylgalactosamine 3-β-galactosyltransferase 1 (C1GALT1) leads to increased expression of these truncated O-glycans. We investigated whether and how truncated O-glycans contributes to the development and progression of PDAC using mice with disruption of C1galt1.
Methods: We crossed C1galt1 floxed mice (C1galt1loxP/loxP) with KrasG12D/+; Trp53R172H/+; Pdx1-Cre (KPC) mice to create KPCC mice. Growth and progression of pancreatic tumors were compared between KPC and KPCC mice; pancreatic tissues were collected and analyzed by immunohistochemistry; immunofluorescence; and Sirius red, alcian blue, and lectin staining. We used the CRISPR/Cas9 system to disrupt C1GALT1 in human PDAC cells (T3M4 and CD18/HPAF) and levels of O-glycans were analyzed by lectin blotting, mass spectrometry, and lectin pulldown assay. Orthotopic studies and RNA sequencing analyses were performed with control and C1GALT1 knockout PDAC cells. C1GALT1 expression was analyzed in well-differentiated (n = 36) and poorly differentiated (n = 23) PDAC samples by immunohistochemistry.
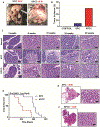
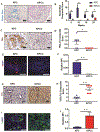
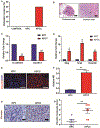
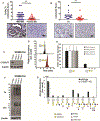
Results: KPCC mice had significantly shorter survival times (median 102 days) than KPC mice (median 200 days) and developed early pancreatic intraepithelial neoplasias at 3 weeks, PDAC at 5 weeks, and metastasis at 10 weeks compared with KPC mice. Pancreatic tumors that developed in KPCC mice were more aggressive (more invasive and metastases) than those in KPC mice, had a decreased amount of stroma, and had increased production of Tn. Poorly differentiated PDAC specimens had significantly lower levels of C1GALT1 than well-differentiated PDACs. Human PDAC cells with knockout of C1GALT1 had aberrant glycosylation of MUC16 compared with control cells and increased expression of genes that regulate tumorigenesis and metastasis.
Conclusions: In studies of KPC mice with disruption of C1galt1, we found that loss of C1galt1 promotes development of aggressive PDACs and increased metastasis. Knockout of C1galt1 leads to increased tumorigenicity and truncation of O-glycosylation on MUC16, which could contribute to increased aggressiveness.
Keywords: Mouse Model; Pancreas; Pancreatic Intraepithelial Neoplasias; Post-Translational Modification.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. - PubMed
-
- Borazanci E, Dang CV, Robey RW, et al. Pancreatic Cancer: “A Riddle Wrapped in a Mystery inside an Enigma”. Clin Cancer Res 2017;23:1629–1637. - PubMed
-
- Leach SD. Mouse models of pancreatic cancer: the fur is finally flying. Cancer Cell 2004;5:7–11. - PubMed
-
- Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumour biology and progressing translational oncology. Gut 2012;61:1488–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

