Mechanochemical ablation versus cyanoacrylate adhesive for the treatment of varicose veins: study protocol for a randomised controlled trial
- PMID: 30086774
- PMCID: PMC6081848
- DOI: 10.1186/s13063-018-2807-0
Mechanochemical ablation versus cyanoacrylate adhesive for the treatment of varicose veins: study protocol for a randomised controlled trial
Abstract
Background: Thermal ablation techniques have become the first-line treatment of truncal veins in the management of chronic venous disease (CVD). Despite excellent outcomes, these methods are often associated with pain; generally due to their use of heat and the necessity of fluid infiltration around the vein. More recently, novel non-thermal techniques, such as mechanochemical ablation (MOCA) and cyanoacrylate adhesive (CAE) have been developed to overcome these unwelcome effects. So far, the novel techniques have been found to have similar efficacy to thermal methods, yet no direct comparisons between the non-thermal treatment techniques have been conducted to date, giving rise to this study.
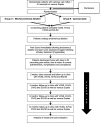
Methods/design: This is a prospective, multicentre, randomised clinical trial, recruiting patients with truncal saphenous incompetence. Patients will be randomised to undergo either MOCA or CAE truncal ablation, followed by treatment of any varicosities. All patients will be required to wear compression stockings for 4 days post intervention. The primary outcome measure is the pain score immediately following completion of truncal ablation, measured by a 100-mm Visual Analogue Scale (VAS). The secondary outcomes are entire treatment pain scores, clinical scores, quality of life scores, occlusion rates, time to return to usual activities/work at 2 weeks, 3, 6 and 12 months. Re-intervention rate will be considered from the third month. Cost-effectiveness will be assessed for each intervention at 12 months. The study is powered to detect a mean 10-mm difference in maximum pain score. Allowing for loss to follow-up, the total target recruitment is 180 patients.
Discussion: The study will be the first study to compare MOCA against CAE and is designed to determine which method causes less pain. Completion of this study is expected to be the end of 2019.
Trial registration: ClinicalTrials.gov, ID: NCT03392753 . Registered on 17 November 2017.
Keywords: Cyanoacrylate adhesive; Endovenous ablation; Mechanochemical ablation; Varicose veins; Venous disease.
Conflict of interest statement
Ethics approval and consent to participate
National Research Ethics Service approval: Regional Committee London: (REC Ref: 17/LO/1457).
Consent for publication
Consent for publication will be sought from each participant at the time of taking consent for participation.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Biemans AAM, Kockaert M, Akkersdijk GP, van den Bos RR, de Maeseneer MGR, Cuypers P, et al. Comparing endovenous laser ablation, foam sclerotherapy, and conventional surgery for great saphenous varicose veins. Journal of Vascular Surgery. 2013;58(3):727–734.e1. doi: 10.1016/j.jvs.2012.12.074. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

