BET Bromodomain Inhibition Cooperates with PD-1 Blockade to Facilitate Antitumor Response in Kras-Mutant Non-Small Cell Lung Cancer
- PMID: 30087114
- PMCID: PMC6170698
- DOI: 10.1158/2326-6066.CIR-18-0077
BET Bromodomain Inhibition Cooperates with PD-1 Blockade to Facilitate Antitumor Response in Kras-Mutant Non-Small Cell Lung Cancer
Abstract
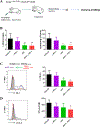
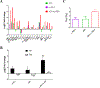
KRAS mutation is present in approximately 30% of human lung adenocarcinomas. Although recent advances in targeted therapy have shown great promise, effective targeting of KRAS remains elusive, and concurrent alterations in tumor suppressors render KRAS-mutant tumors even more resistant to existing therapies. Contributing to the refractoriness of KRAS-mutant tumors are immunosuppressive mechanisms, such as increased presence of suppressive regulatory T cells (Treg) in tumors and elevated expression of the inhibitory receptor PD-1 on tumor-infiltrating T cells. Treatment with BET bromodomain inhibitors is beneficial for hematologic malignancies, and they have Treg-disruptive effects in a non-small cell lung cancer (NSCLC) model. Targeting PD-1-inhibitory signals through PD-1 antibody blockade also has substantial therapeutic impact in lung cancer, although these outcomes are limited to a minority of patients. We hypothesized that the BET bromodomain inhibitor JQ1 would synergize with PD-1 blockade to promote a robust antitumor response in lung cancer. In the present study, using Kras+/LSL-G12D ; Trp53L/L (KP) mouse models of NSCLC, we identified cooperative effects between JQ1 and PD-1 antibody. The numbers of tumor-infiltrating Tregs were reduced and activation of tumor-infiltrating T cells, which had a T-helper type 1 (Th1) cytokine profile, was enhanced, underlying their improved effector function. Furthermore, lung tumor-bearing mice treated with this combination showed robust and long-lasting antitumor responses compared with either agent alone, culminating in substantial improvement in the overall survival of treated mice. Thus, combining BET bromodomain inhibition with immune checkpoint blockade offers a promising therapeutic approach for solid malignancies such as lung adenocarcinoma. Cancer Immunol Res; 6(10); 1234-45. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

