Loss of Function of Canonical Notch Signaling Drives Head and Neck Carcinogenesis
- PMID: 30087145
- PMCID: PMC6295262
- DOI: 10.1158/1078-0432.CCR-17-3535
Loss of Function of Canonical Notch Signaling Drives Head and Neck Carcinogenesis
Abstract
Purpose: Head and neck squamous cell carcinoma (HNSCC), a common cancer worldwide, is etiologically associated with tobacco use, high alcohol consumption, and high-risk human papillomaviruses (HPV). The Notch signaling pathway, which is involved in cell differentiation decisions with differential downstream targets and effects depending on tissue type and developmental stage, has been implicated in human HNSCC. NOTCH1 is among the most frequently mutated genes in both HPV-positive and HPV-negative HNSCC. These mutations are predicted to inactivate the function of Notch. Other studies have argued the opposite-Notch signaling is increased in HNSCC.
Experimental design: To assess the role of Notch signaling in HPV-positive and HPV-negative HNSCC, we utilized genetically engineered mouse (GEM) models for conventional keratinizing HNSCC, in which either HPV16 E6 and E7 oncoproteins or a gain-of-function mutant p53 are expressed, and in which we inactivated canonical Notch signaling via expression of a dominant negative form of MAML1 (DNMAML1), a required transcriptional coactivator of Notch signaling.
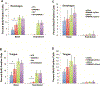
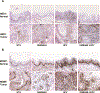
Results: Loss of canonical Notch signaling increased tumorigenesis in both contexts and also caused an increase in nuclear β-catenin, a marker for increased tumorigenic potential. When combined with loss of canonical Notch signaling, HPV oncogenes led to the highest frequency of cancers overall and the largest number of poorly differentiated (high-grade) cancers.
Conclusions: These findings inform on the contribution of loss of canonical Notch signaling in head and neck carcinogenesis.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest
Figures



References
-
- Cancer Facts and Figures. In: American Cancer Society Edited by Society AC. Atlanta, GA; 2011.
-
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L et al.: Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of the National Cancer Institute 2000, 92(9):709–720. - PubMed
-
- Franceschi S, Talamini R, Barra S, Baron AE, Negri E, Bidoli E et al.: Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer research 1990, 50(20):6502–6507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

