Azithromycin Resistance through Interspecific Acquisition of an Epistasis-Dependent Efflux Pump Component and Transcriptional Regulator in Neisseria gonorrhoeae
- PMID: 30087172
- PMCID: PMC6083905
- DOI: 10.1128/mBio.01419-18
Azithromycin Resistance through Interspecific Acquisition of an Epistasis-Dependent Efflux Pump Component and Transcriptional Regulator in Neisseria gonorrhoeae
Abstract
Mosaic interspecifically acquired alleles of the multiple transferable resistance (mtr) efflux pump operon correlate with increased resistance to azithromycin in Neisseria gonorrhoeae in epidemiological studies. However, whether and how these alleles cause resistance is unclear. Here, we use population genomics, transformations, and transcriptional analyses to dissect the relationship between variant mtr alleles and azithromycin resistance. We find that the locus encompassing the mtrR transcriptional repressor and the mtrCDE pump is a hot spot of interspecific recombination introducing alleles from Neisseria meningitidis and Neisseria lactamica into N. gonorrhoeae, with multiple rare haplotypes in linkage disequilibrium at mtrD and the mtr promoter region. Transformations demonstrate that resistance to azithromycin, as well as to other antimicrobial compounds such as polymyxin B and crystal violet, is mediated through epistasis between these two loci and that the full-length mosaic mtrD allele is required. Gene expression profiling reveals the mechanism of resistance in mosaics couples novel mtrD alleles with promoter mutations that increase expression of the pump. Overall, our results demonstrate that epistatic interactions at mtr gained from multiple neisserial species has contributed to increased gonococcal resistance to diverse antimicrobial agents.IMPORTANCENeisseria gonorrhoeae is the sexually transmitted bacterial pathogen responsible for more than 100 million cases of gonorrhea worldwide each year. The incidence of resistance to the macrolide azithromycin has increased in the past decade; however, a large proportion of the genetic basis of resistance remains unexplained. This study is the first to conclusively demonstrate the acquisition of macrolide resistance through mtr alleles from other Neisseria species, demonstrating that commensal Neisseria bacteria are a reservoir for antibiotic resistance to macrolides, extending the role of interspecies mosaicism in resistance beyond what has been previously described for cephalosporins. Ultimately, our results emphasize that future fine-mapping of genome-wide interspecies mosaicism may be valuable in understanding the pathways to antimicrobial resistance. Our results also have implications for diagnostics and public health surveillance and control, as they can be used to inform the development of sequence-based tools to monitor and control the spread of antibiotic-resistant gonorrhea.
Keywords: Neisseria gonorrhoeae; antibiotic resistance; efflux pump; epistasis; gonorrhea; macrolide.
Copyright © 2018 Wadsworth et al.
Figures


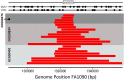




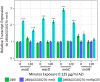
Comment in
-
Mosaic Drug Efflux Gene Sequences from Commensal Neisseria Can Lead to Low-Level Azithromycin Resistance Expressed by Neisseria gonorrhoeae Clinical Isolates.mBio. 2018 Sep 11;9(5):e01747-18. doi: 10.1128/mBio.01747-18. mBio. 2018. PMID: 30206175 Free PMC article.
References
-
- Centers for Disease Control and Prevention 2016. Gonococcal Isolate Surveillance Project (GISP). Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/std/gisp/. Accessed 24 April 2018.
-
- Public Health England 2017. Surveillance of antimicrobial resistance in Neisseria gonorrhoeae in England and Wales. Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP). Antimicrobial resistance in Neisseria gonorrhoeae: data to June 2017, p 1–24. Public Health England, London, United Kingdom: http://www.gov.uk/government/publications/gonococcal-resistance-to-antim....
-
- European Centre for Disease Prevention and Control 2017. Gonococcal antimicrobial susceptibility surveillance in Europe, 2015. ECDC Surveillance Report, p 1–44. European Centre for Disease Prevention and Control, Stockholm, Sweden: http://www.ecdc.europa.eu.
-
- World Health Organization 2017. Gonococcal antimicrobial resistance in the Western Pacific Region, p 1–4. WHO fact sheet. WHO Regional Office for the Western Pacific, Manila, Philippines.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

