CTLA-4 Mediates Inhibitory Function of Mesenchymal Stem/Stromal Cells
- PMID: 30087255
- PMCID: PMC6121442
- DOI: 10.3390/ijms19082312
CTLA-4 Mediates Inhibitory Function of Mesenchymal Stem/Stromal Cells
Abstract
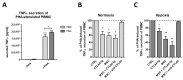
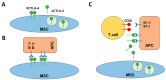
Mesenchymal stem/stromal cells (MSCs) are stem cells of the connective tissue, possess a plastic phenotype, and are able to differentiate into various tissues. Besides their role in tissue regeneration, MSCs perform additional functions as a modulator or inhibitor of immune responses. Due to their pleiotropic function, MSCs have also gained therapeutic importance for the treatment of autoimmune diseases and for improving fracture healing and cartilage regeneration. However, the therapeutic/immunomodulatory mode of action of MSCs is largely unknown. Here, we describe that MSCs express the inhibitory receptor CTLA-4 (cytotoxic T lymphocyte antigen 4). We show that depending on the environmental conditions, MSCs express different isoforms of CTLA-4 with the secreted isoform (sCTLA-4) being the most abundant under hypoxic conditions. Furthermore, we demonstrate that the immunosuppressive function of MSCs is mediated mainly by the secretion of CTLA-4. These findings open new ways for treatment when tissue regeneration/fracture healing is difficult.
Keywords: CTLA-4; fracture healing; hypoxia; immune modulation; mesenchymal stem/stromal cells; regeneration.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Alexander T., Arnold R., Hiepe F., Radbruch A. Resetting the immune system with immunoablation and autologous haematopoietic stem cell transplantation in autoimmune diseases. Clin. Exp. Rheumatol. 2016;34:53–57. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

