Developmental seizures and mortality result from reducing GABAA receptor α2-subunit interaction with collybistin
- PMID: 30087324
- PMCID: PMC6081406
- DOI: 10.1038/s41467-018-05481-1
Developmental seizures and mortality result from reducing GABAA receptor α2-subunit interaction with collybistin
Abstract
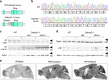
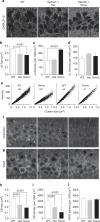
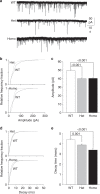
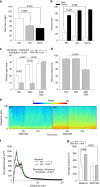
Fast inhibitory synaptic transmission is mediated by γ-aminobutyric acid type A receptors (GABAARs) that are enriched at functionally diverse synapses via mechanisms that remain unclear. Using isothermal titration calorimetry and complementary methods we demonstrate an exclusive low micromolar binding of collybistin to the α2-subunit of GABAARs. To explore the biological relevance of collybistin-α2-subunit selectivity, we generate mice with a mutation in the α2-subunit-collybistin binding region (Gabra2-1). The mutation results in loss of a distinct subset of inhibitory synapses and decreased amplitude of inhibitory synaptic currents. Gabra2-1 mice have a striking phenotype characterized by increased susceptibility to seizures and early mortality. Surviving Gabra2-1 mice show anxiety and elevations in electroencephalogram δ power, which are ameliorated by treatment with the α2/α3-selective positive modulator, AZD7325. Taken together, our results demonstrate an α2-subunit selective binding of collybistin, which plays a key role in patterned brain activity, particularly during development.
Conflict of interest statement
S.J.M. serves as a consultant for SAGE Therapeutics and AstraZeneca, relationships that are regulated by Tufts University. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

