Priming With Intermittent Theta Burst Transcranial Magnetic Stimulation Promotes Spinal Plasticity Induced by Peripheral Patterned Electrical Stimulation
- PMID: 30087593
- PMCID: PMC6066516
- DOI: 10.3389/fnins.2018.00508
Priming With Intermittent Theta Burst Transcranial Magnetic Stimulation Promotes Spinal Plasticity Induced by Peripheral Patterned Electrical Stimulation
Abstract
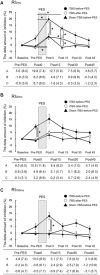
This study explored the effect of corticospinal activity on spinal plasticity by examining the interactions between intermittent theta burst transcranial magnetic stimulation (iTBS) of the motor cortex and peripheral patterned electrical stimulation (PES) of the common peroneal nerve (CPN). Healthy volunteers (n = 10) received iTBS to the tibialis anterior (TA) muscle zone of the motor cortex and PES of the CPN in three separate sessions: (1) iTBS-before-PES, (2) iTBS-after-PES, and (3) sham iTBS-before-PES. The PES protocol used 10 100-Hz pulses every 2 s for 20 min. Reciprocal inhibition (RI) from the TA to soleus muscle and motor cortical excitability of the TA and soleus muscles were assessed at baseline, before PES, and 0, 15, 30, and 45 min after PES. When compared to the other protocols, iTBS-before-PES significantly increased changes in disynaptic RI for 15 min and altered long-loop presynaptic inhibition immediately after PES. Moreover, the iTBS-induced cortical excitability changes in the TA before PES were correlated with the enhancement of disynaptic RI immediately after PES. These results demonstrate that spinal plasticity can be modified by altering cortical excitability. This study provides insight into the interactions between modulation of corticospinal excitability and spinal RI, which may help in developing new rehabilitation strategies.
Keywords: H-reflex; disynaptic reciprocal inhibition; non-invasive brain stimulation; presynaptic inhibition; spinal plasticity.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

