Isolation and Characterization of an Acyclic Isoprenoid from Semecarpus anacardium Linn. and its Antibacterial Potential in vitro: - Antimicrobial Activity of Semecarpus anacardium Linn. Seeds
- PMID: 30087789
- PMCID: PMC5532471
- DOI: 10.3831/KPI.2017.20.016
Isolation and Characterization of an Acyclic Isoprenoid from Semecarpus anacardium Linn. and its Antibacterial Potential in vitro: - Antimicrobial Activity of Semecarpus anacardium Linn. Seeds
Abstract
Objectives: Semecarpus anacardium Linn. is a plant well-known for its antimicrobial, antidiabetic and anti-arthritic properties in the Ayurvedic and Siddha system of medicine. This has prompted the screening of this plant for antibacterial activity. The main aims of this study were to isolate compounds from the plant's seeds and to evaluate their antibacterial effects on clinical bacterial test strains.
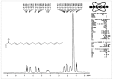
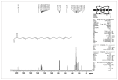
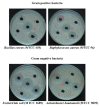
Methods: The n-butanolic concentrate of the seed extract was subjected to thin layer chromatography (TLC) and repeated silica gel column chromatography followed by elution with various solvents. The compound was identified based on observed spectral (IR, 1H NMR, 13C NMR and high-resolution mass spectrometry) data. The well diffusion method was employed to evaluate the antibacterial activities of the isolated acyclic isoprenoid compound (final concentration: 5 - 15 μg/mL) on four test bacterial strains, namely, Staphylococcus aureus (MTCC 96), Bacillus cereus (MTCC 430), Escherichia coli (MTCC 1689) and Acinetobacter baumannii (MTCC 9829).
Results: Extensive spectroscopic studies showed the structure of the isolated compound to be an acyclic isoprenoid (C21H32O). Moreover, the isoprenoid showed a remarkable inhibition of bacterial growth at a concentration of 15 μg/mL compared to the two other doses tested (5 and 10 μg/mL) and to tetracycline, a commercially available antibiotic that was used as a reference drug.
Conclusion: The isolation of an antimicrobial compound from Semecarpus anacardium seeds validates the use of this plant in the treatment of infections. The isolated compound found to be active in this study could be useful for the development of new antimicrobial drugs.
Keywords: Semecarpus anacardium Linn; acyclic isoprenoid; antibacterial; disk diffusion method; high-resolution mass spectrometry.
Conflict of interest statement
Conflict of interest The authors declare that there are no conflicts of interest.
Figures




References
-
- Heinrich M, Barnes J, Gibbons S, Williamson E. Fundamentals of pharmacognosy and phytotherapy. London: Elsevier Science Ltd; 2004. p. 336.
-
- Chopra RN. Chopra’s indigenous drugs of India. Kolkata: Calcutta Academic Publishers; 1982. pp. 407–9.
-
- Khare CP. Encyclopedia of Indian medicinal plants. Germany: Springer-Verlag; 1982. pp. 419–21.
LinkOut - more resources
Full Text Sources
