Analysis and Isolation of Potential Artemisinin Precursors from Waste Streams of Artemisia Annua Extraction
- PMID: 30087924
- PMCID: PMC6068693
- DOI: 10.1021/acsomega.8b00974
Analysis and Isolation of Potential Artemisinin Precursors from Waste Streams of Artemisia Annua Extraction
Abstract
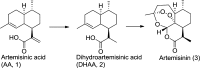
High-performance liquid chromatography, liquid chromatography-mass spectrometry, and gas chromatography-mass spectrometry methods were developed to analyze the process waste streams of Artemisia Annua extraction. Results from these methods suggested that the final waste from the extraction process could serve as a source of dihydroartemisinic acid (DHAA) that could be converted to additional artemisinin. Two additional impurities were isolated and identified in the waste material as well as in A. annua leaf samples. That these impurities also appear as side-products in chemical transformations of DHAA to artemisinin supports the conclusion that the in vivo transformation proceeds as nonspecific oxidations. These impurities do not appear in isolated artemisinin. A simple, high-yielding procedure for recovery of DHAA from the primary waste stream was developed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Ro D.-K.; Paradise E. M.; Ouellet M.; Fisher K. L.; Newman K. L.; Ndungu J. M.; Ho K. A.; Eachus R. A.; Ham T. S.; Kirby J.; Chang M. C. Y.; Withers S. T.; Shiba Y.; Sarpong R.; Keasling J. D. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. 10.1038/nature04640. - DOI - PubMed
-
- Turconi J.; Griolet F.; Guevel R.; Oddon G.; Villa R.; Geatti A.; Hvala M.; Rossen K.; Göller R.; Burgard A. Semisynthetic artemisinin, the chemical path to industrial production. Org. Process Res. Dev. 2014, 18, 417–422. 10.1021/op4003196. - DOI
-
- Yadav J. S.; Thirupathaiah B.; Srihari P. A concise stereoselective total synthesis of (+)-artemisinin. Tetrahedron 2010, 66, 2005–2009. 10.1016/j.tet.2010.01.051. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources