Long-Lasting Androgen-Induced Cardiometabolic Effects in Polycystic Ovary Syndrome
- PMID: 30087950
- PMCID: PMC6065488
- DOI: 10.1210/js.2018-00131
Long-Lasting Androgen-Induced Cardiometabolic Effects in Polycystic Ovary Syndrome
Abstract
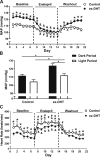
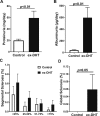
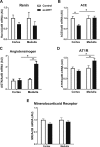
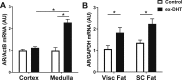
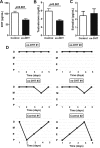
Polycystic ovary syndrome (PCOS), the most common endocrine disorder in women of reproductive age, is characterized by androgen excess and ovarian dysfunction and presents with increased cardiometabolic risk factors such as obesity, insulin resistance, and elevated blood pressure (BP). We previously reported that administration of dihydrotestosterone (DHT) to female rats elicits cardiometabolic derangements similar to those found in women with PCOS. In this study, we tested the hypothesis that the DHT-mediated cardiometabolic derangements observed in PCOS are long lasting despite DHT withdrawal. Four-week-old female Sprague Dawley rats were treated with DHT (7.5 mg/90 days) or placebo for 6 months. DHT was discontinued (ex-DHT), and rats were followed for 6 additional months. After 6 months of DHT withdrawal, food intake, body weight, fat and lean mass, fasting plasma insulin, leptin, and adiponectin were elevated in ex-DHT rats. BP remained significantly elevated, and enalapril, an angiotensin-converting enzyme (ACE) inhibitor, normalized BP in ex-DHT rats. Expression of components of the intrarenal renin-angiotensin system was increased in ex-DHT rats. The cardiometabolic features found in ex-DHT rats were associated with lower plasma androgen levels but increased expression of renal and adipose tissue androgen receptors. In summary, androgen-induced cardiometabolic effects persisted after DHT withdrawal in a PCOS experimental model. Activation of intrarenal renin-angiotensin system plays a major role in the androgen-mediated increase in BP in ex-DHT. Upregulation of the renal and adipose tissue androgen receptor may explain the long-lasting effects of androgens. In clinical scenarios characterized by hyperandrogenemia in women, prompt normalization of androgen levels may be necessary to prevent their long-lasting cardiometabolic effects.
Keywords: androgen receptor; androgens; blood pressure; cardiometabolic risk factors; polycystic ovary syndrome; renin angiotensin system.
Figures

References
-
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855. - PubMed
-
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. - PubMed
-
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. - PubMed
-
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
