Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth
- PMID: 30089075
- PMCID: PMC6004541
- DOI: 10.1056/NEJMoa1800927
Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth
Erratum in
-
Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth.N Engl J Med. 2021 Oct 28;385(18):1728. doi: 10.1056/NEJMx210014. N Engl J Med. 2021. PMID: 34706188 No abstract available.
Abstract
Background: It is unclear whether maternal vitamin D supplementation during pregnancy and lactation improves fetal and infant growth in regions where vitamin D deficiency is common.
Methods: We conducted a randomized, double-blind, placebo-controlled trial in Bangladesh to assess the effects of weekly prenatal vitamin D supplementation (from 17 to 24 weeks of gestation until birth) and postpartum vitamin D supplementation on the primary outcome of infants' length-for-age z scores at 1 year according to World Health Organization (WHO) child growth standards. One group received neither prenatal nor postpartum vitamin D (placebo group). Three groups received prenatal supplementation only, in doses of 4200 IU (prenatal 4200 group), 16,800 IU (prenatal 16,800 group), and 28,000 IU (prenatal 28,000 group). The fifth group received prenatal supplementation as well as 26 weeks of postpartum supplementation in the amount of 28,000 IU (prenatal and postpartum 28,000 group).
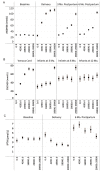
Results: Among 1164 infants assessed at 1 year of age (89.5% of 1300 pregnancies), there were no significant differences across groups in the mean (±SD) length-for-age z scores. Scores were as follows: placebo, -0.93±1.05; prenatal 4200, -1.11±1.12; prenatal 16,800, -0.97±0.97; prenatal 28,000, -1.06±1.07; and prenatal and postpartum 28,000, -0.94±1.00 (P=0.23 for a global test of differences across groups). Other anthropometric measures, birth outcomes, and morbidity did not differ significantly across groups. Vitamin D supplementation had expected effects on maternal and infant serum 25-hydroxyvitamin D and calcium concentrations, maternal urinary calcium excretion, and maternal parathyroid hormone concentrations. There were no significant differences in the frequencies of adverse events across groups, with the exception of a higher rate of possible hypercalciuria among the women receiving the highest dose.
Conclusions: In a population with widespread prenatal vitamin D deficiency and fetal and infant growth restriction, maternal vitamin D supplementation from midpregnancy until birth or until 6 months post partum did not improve fetal or infant growth. (Funded by the Bill and Melinda Gates Foundation; ClinicalTrials.gov number, NCT01924013 .).
Figures

Comment in
-
Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth.N Engl J Med. 2018 Nov 8;379(19):1880-1. doi: 10.1056/NEJMc1812157. N Engl J Med. 2018. PMID: 30406981 No abstract available.
References
-
- Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med 2016; 50: 761-79. - PubMed
-
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382: 427-51. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
