Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial
- PMID: 30089078
- PMCID: PMC6145832
- DOI: 10.1200/JCO.2018.79.1483
Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial
Abstract
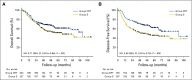
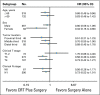
Purpose The efficacy of neoadjuvant chemoradiotherapy (NCRT) plus surgery for locally advanced esophageal squamous cell carcinoma (ESCC) remains controversial. In this trial, we compared the survival and safety of NCRT plus surgery with surgery alone in patients with locally advanced ESCC. Patients and Methods From June 2007 to December 2014, 451 patients with potentially resectable thoracic ESCC, clinically staged as T1-4N1M0/T4N0M0, were randomly allocated to NCRT plus surgery (group CRT; n = 224) and surgery alone (group S; n = 227). In group CRT, patients received vinorelbine 25 mg/m2 intravenously (IV) on days 1 and 8 and cisplatin 75 mg/m2 IV day 1, or 25 mg/m2 IV on days 1 to 4 every 3 weeks for two cycles, with a total concurrent radiation dose of 40.0 Gy administered in 20 fractions of 2.0 Gy on 5 days per week. In both groups, patients underwent McKeown or Ivor Lewis esophagectomy. The primary end point was overall survival. Results The pathologic complete response rate was 43.2% in group CRT. Compared with group S, group CRT had a higher R0 resection rate (98.4% v 91.2%; P = .002), a better median overall survival (100.1 months v 66.5 months; hazard ratio, 0.71; 95% CI, 0.53 to 0.96; P = .025), and a prolonged disease-free survival (100.1 months v 41.7 months; hazard ratio, 0.58; 95% CI, 0.43 to 0.78; P < .001). Leukopenia (48.9%) and neutropenia (45.7%) were the most common grade 3 or 4 adverse events during chemoradiotherapy. Incidences of postoperative complications were similar between groups, with the exception of arrhythmia (group CRT: 13% v group S: 4.0%; P = .001). Peritreatment mortality was 2.2% in group CRT versus 0.4% in group S ( P = .212). Conclusion This trial shows that NCRT plus surgery improves survival over surgery alone among patients with locally advanced ESCC, with acceptable and manageable adverse events.
Trial registration: ClinicalTrials.gov NCT01216527.
Figures
Comment in
-
Editorial comments for neoadjuvant chemo-radiotherapy in the treatment of locally advanced squamous cell esophageal cancer.J Thorac Dis. 2018 Nov;10(11):5979-5981. doi: 10.21037/jtd.2018.10.81. J Thorac Dis. 2018. PMID: 30622767 Free PMC article. No abstract available.
-
Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus: the (NEOCRTEC5010) trial-a timely and welcome clinical trial from the Far East.J Thorac Dis. 2018 Nov;10(Suppl 33):S4162-S4164. doi: 10.21037/jtd.2018.10.39. J Thorac Dis. 2018. PMID: 30631582 Free PMC article. No abstract available.
-
Interpreting the Survival Benefit From Neoadjuvant Chemoradiotherapy Before Surgery for Locally Advanced Squamous Cell Carcinoma of the Esophagus.J Clin Oncol. 2019 Apr 20;37(12):1032-1033. doi: 10.1200/JCO.18.01164. Epub 2019 Mar 6. J Clin Oncol. 2019. PMID: 30840522 No abstract available.
-
Reply to Z. McCaw and L-J. Wei.J Clin Oncol. 2019 Apr 20;37(12):1034. doi: 10.1200/JCO.18.01829. Epub 2019 Mar 6. J Clin Oncol. 2019. PMID: 30840523 No abstract available.
-
Gastrointestinal Cancers: Fine-Tuning the Management of Rectal, Esophageal, and Pancreas Cancers.Int J Radiat Oncol Biol Phys. 2019 Sep 1;105(1):1-10. doi: 10.1016/j.ijrobp.2019.04.037. Int J Radiat Oncol Biol Phys. 2019. PMID: 31422802 Free PMC article. No abstract available.
References
-
- Jemal A, Bray F, Center MM, et al. : Global cancer statistics. CA Cancer J Clin 61:69-90, 2011 - PubMed
-
- Chen W, Zheng R, Baade PD, et al. : Cancer statistics in China, 2015. CA Cancer J Clin 66:115-132, 2016 - PubMed
-
- Herskovic A, Russell W, Liptay M, et al. : Esophageal carcinoma advances in treatment results for locally advanced disease: Review. Ann Oncol 23:1095-1103, 2012 - PubMed
-
- Sjoquist KM, Burmeister BH, Smithers BM, et al. : Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol 12:681-692, 2011 - PubMed
-
- Apinop C, Puttisak P, Preecha N: A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology 41:391-393, 1994 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

