Proteasome inhibition induces IKK-dependent interleukin-8 expression in triple negative breast cancer cells: Opportunity for combination therapy
- PMID: 30089134
- PMCID: PMC6082561
- DOI: 10.1371/journal.pone.0201858
Proteasome inhibition induces IKK-dependent interleukin-8 expression in triple negative breast cancer cells: Opportunity for combination therapy
Abstract
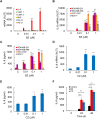
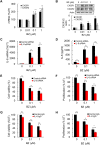
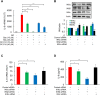
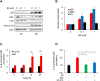
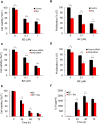
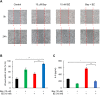
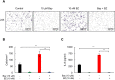
Triple negative breast cancer (TNBC) cells express increased levels of the pro-inflammatory and pro-angiogenic chemokine interleukin-8 (IL-8, CXCL8), which promotes their proliferation and migration. Because TNBC patients are unresponsive to current targeted therapies, new therapeutic strategies are urgently needed. While proteasome inhibition by bortezomib (BZ) or carfilzomib (CZ) has been effective in treating hematological malignancies, it has been less effective in solid tumors, including TNBC, but the mechanisms are incompletely understood. Here we report that proteasome inhibition significantly increases expression of IL-8, and its receptors CXCR1 and CXCR2, in TNBC cells. Suppression or neutralization of the BZ-induced IL-8 potentiates the BZ cytotoxic and anti-proliferative effect in TNBC cells. The IL-8 expression induced by proteasome inhibition in TNBC cells is mediated by IκB kinase (IKK), increased nuclear accumulation of p65 NFκB, and by IKK-dependent p65 recruitment to IL-8 promoter. Importantly, inhibition of IKK activity significantly decreases proliferation, migration, and invasion of BZ-treated TNBC cells. These data provide the first evidence demonstrating that proteasome inhibition increases the IL-8 signaling in TNBC cells, and suggesting that IKK inhibitors may increase effectiveness of proteasome inhibitors in treating TNBC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

