Antenatal IL-1-dependent inflammation persists postnatally and causes retinal and sub-retinal vasculopathy in progeny
- PMID: 30089839
- PMCID: PMC6082873
- DOI: 10.1038/s41598-018-30087-4
Antenatal IL-1-dependent inflammation persists postnatally and causes retinal and sub-retinal vasculopathy in progeny
Erratum in
-
Author Correction: Antenatal IL-1-dependent inflammation persists postnatally and causes retinal and sub-retinal vasculopathy in progeny.Sci Rep. 2020 Apr 15;10(1):6634. doi: 10.1038/s41598-020-63705-1. Sci Rep. 2020. PMID: 32296110 Free PMC article.
Abstract
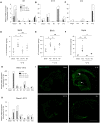
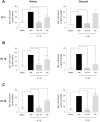
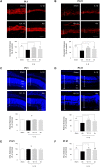
Antenatal inflammation as seen with chorioamnionitis is harmful to foetal/neonatal organ development including to eyes. Although the major pro-inflammatory cytokine IL-1β participates in retinopathy induced by hyperoxia (a predisposing factor to retinopathy of prematurity), the specific role of antenatal IL-1β associated with preterm birth (PTB) in retinal vasculopathy (independent of hyperoxia) is unknown. Using a murine model of PTB induced with IL-1β injection in utero, we studied consequent retinal and choroidal vascular development; in this process we evaluated the efficacy of IL-1R antagonists. Eyes of foetuses exposed only to IL-1β displayed high levels of pro-inflammatory genes, and a persistent postnatal infiltration of inflammatory cells. This prolonged inflammatory response was associated with: (1) a marked delay in retinal vessel growth; (2) long-lasting thinning of the choroid; and (3) long-term morphological and functional alterations of the retina. Antenatal administration of IL-1R antagonists - 101.10 (a modulator of IL-1R) more so than Kineret (competitive IL-1R antagonist) - prevented all deleterious effects of inflammation. This study unveils a key role for IL-1β, a major mediator of chorioamnionitis, in causing sustained ocular inflammation and perinatal vascular eye injury, and highlights the efficacy of antenatal 101.10 to suppress deleterious inflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Institute of Medicine Committee on Understanding Premature, B. & Assuring Healthy, O. In Preterm Birth: Causes, Consequences, and Prevention (eds Behrman, R. E. & Butler, A. S.) (National Academies Press (US) National Academy of Sciences., 2007). - PubMed
-
- Liu L, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet (London, England) 2015;385:430–440. - PubMed
-
- Verstraeten BS, Mijovic-Kondejewski J, Takeda J, Tanaka S, Olson DM. Canada’s pregnancy-related mortality rates: doing well but room for improvement. Clinical and investigative medicine. Medecine clinique et experimentale. 2015;38:E15–22. - PubMed
-
- ACOG committee opinion no. 561 Nonmedically indicated early-term deliveries. Obstetrics and gynecology. 2013;121:911–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

