Catalytic two-electron reduction of dioxygen catalysed by metal-free [14]triphyrin(2.1.1)
- PMID: 30090268
- PMCID: PMC6054055
- DOI: 10.1039/c5sc02465j
Catalytic two-electron reduction of dioxygen catalysed by metal-free [14]triphyrin(2.1.1)
Abstract
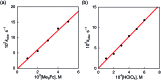
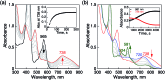
The catalytic two-electron reduction of dioxygen (O2) by octamethylferrocene (Me8Fc) occurs with a metal-free triphyrin (HTrip) in the presence of perchloric acid (HClO4) in benzonitrile (PhCN) at 298 K to yield Me8Fc+ and H2O2. Detailed kinetic analysis has revealed that the catalytic two-electron reduction of O2 by Me8Fc with HTrip proceeds via proton-coupled electron transfer from Me8Fc to HTrip to produce H3Trip˙+, followed by a second electron transfer from Me8Fc to H3Trip˙+ to produce H3Trip, which is oxidized by O2via formation of the H3Trip/O2 complex to yield H2O2. The rate-determining step in the catalytic cycle is hydrogen atom transfer from H3Trip to O2 in the H3Trip/O2 complex to produce the radical pair (H3Trip˙+ HO2˙) as an intermediate, which was detected as a triplet EPR signal with fine-structure by the EPR measurements at low temperature. The distance between the two unpaired electrons in the radical pair was determined to be 4.9 Å from the zero-field splitting constant (D).
Figures






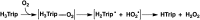




References
-
- Abrantes S., Amaral E., Costa A. P., Shatalov A. A., Duarte A. P. Ind. Crops Prod. 2007;25:288–293.
- Zeronian S. H., Inglesby M. K. Cellulose. 1995;2:265–272.
-
- Li L., Lee S., Lee H. L., Youn H. J. BioResources. 2011;6:721–736.
-
- Yamazaki S., Siroma Z., Senoh H., Ioroi T., Fujiwara N., Yasuda K. J. Power Sources. 2008;178:20–25.
- Disselkamp R. S. Energy Fuels. 2008;22:2771–2774.
- Disselkamp R. S. Int. J. Hydrogen Energy. 2010;35:1049–1053.
-
- Yamada Y., Fukuzumi S. Aust. J. Chem. 2014;67:354–364.
- Yamada Y., Yoneda M., Fukuzumi S. Inorg. Chem. 2014;53:1272–1274. - PubMed
- Yamada Y., Yoneda M., Fukuzumi S. Chem.–Eur. J. 2013;19:11733–11741. - PubMed
- Yamada Y., Yoshida S., Honda T., Fukuzumi S. Energy Environ. Sci. 2011;4:2822–2825.
- Yamada Y., Fukunishi Y., Yamazaki S., Fukuzumi S. Chem. Commun. 2010;46:7334–7336. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

