A DEHP plasticizer alters synaptic proteins via peroxidation
- PMID: 30090480
- PMCID: PMC6060737
- DOI: 10.1039/c6tx00361c
A DEHP plasticizer alters synaptic proteins via peroxidation
Abstract
Di-(2-ethylhexyl) phthalate (DEHP) is a widely used commercial plasticizer. DEHP exposure has a negative impact on brain development and cognition, but the mechanisms responsible for DEHP-induced neurotoxicity are not well understood. Here we showed that DEHP exposure increased maleic dialdehyde and reactive oxygen species contents and decreased endogenous superoxide dismutase activity in a mouse neuroblastoma cell line (N2a cell line). DEHP exposure not only induced reduction of neurite outgrowth, but also led to microtubule-associated protein tau hyperphosphorylation and dissociation from microtubules. Furthermore, DEHP exposure decreased the levels of synapsin-1 and postsynaptic density protein 95 (PSD95), which play critical roles in synaptic function. Antioxidant vitamin E pretreatment prevented DEHP-induced abnormalities in the cells. These results indicate that DEHP exposure could induce abnormal action of proteins including tau, synapsin-1 and PSD95, which play critical roles in the synaptic structure and function, and that these alterations might be mediated by peroxidative damage.
Figures



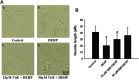
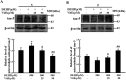



References
-
- Anderson D., Yu T. W., Hincal F. Teratog., Carcinog., Mutagen. 1999;19:275–280. - PubMed
-
- Rubin R. J., Schiffer C. A. Transfusion. 1976;16:330–335. - PubMed
-
- Hashizume K., Nanya J., Toda C., Yasui T., Nagano H., Kojima N. Biol. Pharm. Bull. 2002;25:209–214. - PubMed
-
- Rudel R. A., Camann D. E., Spengler J. D., Korn L. R., Brody J. G. Environ. Sci. Technol. 2003;37:4543–4553. - PubMed
-
- Chen T., Yang W., Li Y., Chen X., Xu S. Toxicol. Lett. 2011;201:34–41. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

