Bifenthrin-induced neurotoxicity in rats: involvement of oxidative stress
- PMID: 30090562
- PMCID: PMC6062383
- DOI: 10.1039/c7tx00205j
Bifenthrin-induced neurotoxicity in rats: involvement of oxidative stress
Abstract
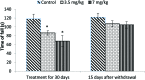
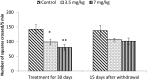
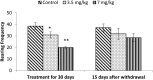
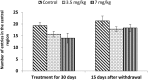
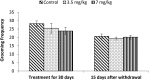
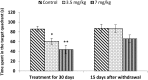
Extensive use of synthetic pyrethroids has resulted in serious human health issues. Induction of oxidative stress is an important mechanism of action of most pesticides including pyrethroids. In the present study, we have elucidated the possible role of oxidative stress in bifenthrin-induced neurotoxicity. Adult male Wistar rats were administered bifenthrin (3.5 and 7 mg per kg body weight p.o.) for 30 days. Behavioral studies were conducted on a set of randomly selected rats from each treatment group after completion of treatment. Neurochemical parameters were assessed 24 h after the last dose was administered. The selected behavioral and neurochemical endpoints were also assessed 15 days after cessation of exposure to reveal whether the neurobehavioral changes produced by bifenthrin were temporary or permanent. Deficits in motor activity, motor incoordination, and cognitive impairment were observed after exposure to bifenthrin. Levels of biogenic amines viz. dopamine (DA) and its metabolites, i.e. 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), epinephrine (EPN), norepinephrine (NE), and serotonin (5-HT) altered in the frontal cortex, corpus striatum, and hippocampus of bifenthrin-treated rats. A decrease in the activity of acetylcholinesterase (AChE) occurred in all regions of the brain. Both doses of bifenthrin significantly induced lipid peroxidation (LPO) and increased protein carbonyl levels in the frontal cortex, corpus striatum, and hippocampus of rats. The activities of antioxidant enzymes, i.e. catalase, superoxide dismutase, and glutathione peroxidase, were also suppressed in all selected regions of the brain. A trend of recovery was, however, observed in all the behavioral and neurochemical endpoints 15 days after withdrawal of exposure. Oxidative stress seems to play an important role in bifenthrin-induced neurotoxicity. Our study suggests that long-term exposure to these compounds can produce detrimental effects.
Figures






References
-
- Gupta S., Sharma R. K., Gupta R. K., Sinha S. R., Singh R., Gajhbhiye V. T. Bull. Environ. Contam. Toxicol. 2009;82:243–247. - PubMed
-
- Mokrey L. E., Hoagland K. D. Environ. Toxicol. Chem. 1989;9:1045–1051.
-
- W.H.O., The WHO recommended classification of pesticides by hazard and guidelines to classification, World Health Organization, Geneva, 2009.
-
- Nguyen H. T., Whelan P. I., Shortus M. S., Jacups S. P. J. Am. Mosq. Control Assoc. 2009;25:74–82. - PubMed
-
- Domagalski J. L., Weston D. P., Zhang M., Hladik M. Environ. Toxicol. Chem. 2010;29:813–823. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

