The subchronic exposure to malathion, an organophosphate pesticide, causes lipid peroxidation, oxidative stress, and tissue damage in rats: the protective role of resveratrol
- PMID: 30090600
- PMCID: PMC6062150
- DOI: 10.1039/c8tx00030a
The subchronic exposure to malathion, an organophosphate pesticide, causes lipid peroxidation, oxidative stress, and tissue damage in rats: the protective role of resveratrol
Abstract
The present study was planned to evaluate the protective role of resveratrol (Res) against subchronic malathion exposure in rats over four weeks. In total, 48 Wistar rats were used and divided equally into six groups. The groups were designed as the control group (received only a rodent diet and tap water), the corn oil group (0.5 ml corn oil by the oral route), and the malathion group (100 mg kg-1 day-1 by the oral route). Other three groups received malathion (100 mg kg-1 day-1) plus Res (5, 10, and 20 mg kg-1 day-1, respectively) by the oral route. Malathion increased malondialdehyde and 8-OHdG levels, whereas it decreased glutathione levels. Also, acetylcholinesterase, superoxide dismutase, and catalase activities were found to be low in the blood, liver, kidney, heart, and brain tissues. Biochemical parameters were not notably changed in all groups. In contrast, Res treatment inverted malathion-induced oxidative stress, lipid peroxidation, and activity of enzymes. Additionally, malathion-induced histopathological changes in the liver, kidney, heart, and brain were ameliorated by Res treatment. These results demonstrate that malathion increases oxidative stress and decreases the antioxidant status while Res has a protective function against malathion toxicity in rats.
Figures
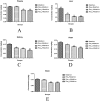
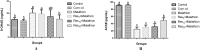

References
-
- El-Demerdash F. M., Nasr H. M. J. Trace Elem. Med. Biol. 2014;28:89–93. - PubMed
-
- Gupta V. K., Sharma B. Anatomy Physiol. Biochem. Int. J. 2016;1:555558.
-
- Suresh B. N., Malik J. K., Rao G. S., Aggarwal M., Ranganathan V. Environ. Toxicol. Pharmacol. 2006;22:167–171. - PubMed
-
- Ince S., Arslan-Acaroz D., Demirel H. H., Varol N., Ozyurek H. A., Zemheri F., Eryavuz A. Biomed Pharmacother. 2017;96:263–268. - PubMed
-
- El-Demerdash F. M. Food Chem. Toxicol. 2011;49:1346–1352. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

