Cerebrospinal Fluid-Directed rAAV9-rsATP7A Plus Subcutaneous Copper Histidinate Advance Survival and Outcomes in a Menkes Disease Mouse Model
- PMID: 30090842
- PMCID: PMC6080355
- DOI: 10.1016/j.omtm.2018.07.002
Cerebrospinal Fluid-Directed rAAV9-rsATP7A Plus Subcutaneous Copper Histidinate Advance Survival and Outcomes in a Menkes Disease Mouse Model
Abstract
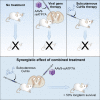
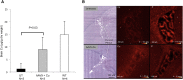
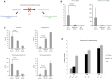
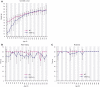
Menkes disease is a lethal neurodegenerative disorder of copper metabolism caused by mutations in an evolutionarily conserved copper transporter, ATP7A. Based on our prior clinical and animal studies, we seek to develop a therapeutic approach suitable for application in affected human subjects, using the mottled-brindled (mo-br) mouse model that closely mimics the Menkes disease biochemical and clinical phenotypes. Here, we evaluate the efficacy of low-, intermediate-, and high-dose recombinant adeno-associated virus serotype 9 (rAAV9)-ATP7A delivered to the cerebrospinal fluid (CSF), in combination with subcutaneous administration of clinical-grade copper histidinate (sc CuHis, IND #34,166). Mutant mice that received high-dose (1.6 × 1010 vg) cerebrospinal fluid-directed rAAV9-rsATP7A plus sc copper histidinate showed 53.3% long-term (≥300-day) survival compared to 0% without treatment or with either treatment alone. The high-dose rAAV9-rsATP7A plus sc copper histidinate-treated mutant mice showed increased brain copper levels, normalized brain neurochemical levels, improvement of brain mitochondrial abnormalities, and normal growth and neurobehavioral outcomes. This synergistic treatment effect represents the most successful rescue to date of the mo-br mouse model. Based on these findings, and the absence of a large animal model, we propose cerebrospinal fluid-directed rAAV9-rsATP7A gene therapy plus subcutaneous copper histidinate as a potential therapeutic approach to cure or ameliorate Menkes disease.
Keywords: ATP7A; Menkes disease; adeno-associated virus; choroid plexus epithelia; copper; dopamine-beta-hydroxylase.
Figures



References
-
- Menkes J.H., Alter M., Steigleder G.K., Weakley D.R., Sung J.H. A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics. 1962;29:764–779. - PubMed
-
- Danks D.M., Campbell P.E., Walker-Smith J., Stevens B.J., Gillespie J.M., Blomfield J., Turner B. Menkes’ kinky-hair syndrome. Lancet. 1972;1:1100–1102. - PubMed
-
- Vulpe C., Levinson B., Whitney S., Packman S., Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 1993;3:7–13. - PubMed
-
- Chelly J., Tümer Z., Tønnesen T., Petterson A., Ishikawa-Brush Y., Tommerup N., Horn N., Monaco A.P. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat. Genet. 1993;3:14–19. - PubMed
-
- Mercer J.F., Livingston J., Hall B., Paynter J.A., Begy C., Chandrasekharappa S., Lockhart P., Grimes A., Bhave M., Siemieniak D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet. 1993;3:20–25. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

