First-in-human study of 177Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer
- PMID: 30090965
- PMCID: PMC6250576
- DOI: 10.1007/s00259-018-4096-y
First-in-human study of 177Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer
Abstract
Purpose: This translational study is designed to assess the safety, dosimetry and therapeutic response to a single, low-dose of 177Lu-EB-PSMA-617 in comparison to 177Lu-PSMA-617 in patients with mCRPC.
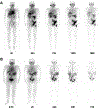
Methods: Following institutional review board approval and informed consent, nine patients with mCRPC were recruited. Four patients accepted intravenous injection of 0.80-1.1 GBq (21.5-30 mCi) of 177Lu-EB-PSMA-617, then underwent serial whole-body planar and SPECT/CT imaging at 2, 24, 72, 120 and 168 h. The other five patients accepted intravenous injection of 1.30-1.42 GBq (35-38.4 mCi) 177Lu-PSMA-617, then underwent the same imaging procedures at 0.5, 2, 24, 48, and 72 h. All patients were evaluated by 68Ga-PSMA-617 PET/CT before and one month after the treatment. Dosimetry evaluation was compared in both patient groups.
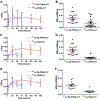
Results: When the bone metastasis tumors with comparable baseline SUVmax in the range of 10.0-15.0 were selected from the two groups for comparison, the accumulated radioactivity of 177Lu-EB-PSMA-617 was about 3.02-fold higher than that of 177Lu-PSMA-617. Imaging dose of 177Lu-EB-PSMA-617 treatment showed significant decrease of 68Ga-PSMA-617 uptake within a month, which was not observed in patients imaged with 177Lu-PSMA-617 (SUV change: -32.43 ± 0.14% vs. 0.21 ± 0.37%; P = 0.002). 177Lu-EB-PSMA-617 also had higher absorbed doses in the red bone marrow and kidneys than 177Lu-PSMA-617 (0.0547 ± 0.0062 vs. 0.0084 ± 0.0057 mSv/MBq for red bone marrow, P < 0.01; 2.39 ± 0.69 vs. 0.39 ± 0.06 mSv/MBq for kidneys, P < 0.01).
Conclusion: This first-in-human study demonstrated that 177Lu-EB-PSMA-617 had higher accumulation in mCRPC and that low imaging dose appears to be effective in treating tumors with high 68Ga-PSMA-617 uptakes. Elevated uptakes of 177Lu-EB-PSMA-617 in kidneys and red bone marrow were well tolerated at the administered low dose. Further investigations with increased dose and frequency of administration are warranted.
Keywords: 177Lu; Evans blue; Metastatic castration-resistant prostate cancer (mCRPC); Prostate-specific membrane antigen (PSMA); Radioligand therapy (RLT).
Conflict of interest statement
Compliance with Ethical Standards
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin, 2017;67(1):7–30. - PubMed
-
- Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192. - PubMed
-
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on PC. Part II: treatment of relapsing, metastatic, and CRPC. Eur Urol. 2017;71:630–42. - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent—update 2013. Eur Urol. 2014;65:124–137. - PubMed
-
- Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol 2005; 288: C975–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

