Human fetoplacental arterial and venous endothelial cells are differentially programmed by gestational diabetes mellitus, resulting in cell-specific barrier function changes
- PMID: 30091044
- PMCID: PMC6182654
- DOI: 10.1007/s00125-018-4699-7
Human fetoplacental arterial and venous endothelial cells are differentially programmed by gestational diabetes mellitus, resulting in cell-specific barrier function changes
Abstract
Aims/hypothesis: An adverse intrauterine environment can result in permanent changes in the physiology of the offspring and predispose to diseases in adulthood. One such exposure, gestational diabetes mellitus (GDM), has been linked to development of metabolic disorders and cardiovascular disease in offspring. Epigenetic variation, including DNA methylation, is recognised as a leading mechanism underpinning fetal programming and we hypothesised that this plays a key role in fetoplacental endothelial dysfunction following exposure to GDM. Thus, we conducted a pilot epigenetic study to analyse concordant DNA methylation and gene expression changes in GDM-exposed fetoplacental endothelial cells.
Methods: Genome-wide methylation analysis of primary fetoplacental arterial endothelial cells (AEC) and venous endothelial cells (VEC) from healthy pregnancies and GDM-complicated pregnancies in parallel with transcriptome analysis identified methylation and expression changes. Most-affected pathways and functions were identified by Ingenuity Pathway Analysis and validated using functional assays.
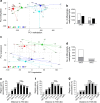
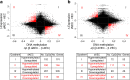
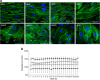
Results: Transcriptome and methylation analyses identified variation in gene expression linked to GDM-associated DNA methylation in 408 genes in AEC and 159 genes in VEC, implying a direct functional link. Pathway analysis found that genes altered by exposure to GDM clustered to functions associated with 'cell morphology' and 'cellular movement' in healthy AEC and VEC. Further functional analysis demonstrated that GDM-exposed cells had altered actin organisation and barrier function.
Conclusions/interpretation: Our data indicate that exposure to GDM programs atypical morphology and barrier function in fetoplacental endothelial cells by DNA methylation and gene expression change. The effects differ between AEC and VEC, indicating a stringent cell-specific sensitivity to adverse exposures associated with developmental programming in utero.
Data availability: DNA methylation and gene expression datasets generated and analysed during the current study are available at the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database ( http://www.ncbi.nlm.nih.gov/geo ) under accession numbers GSE106099 and GSE103552, respectively.
Keywords: Actin organisation; DNA methylation; Fetoplacental endothelial cells; Gestational diabetes mellitus; Programming.
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

