Clinical-grade mesenchymal stem cells derived from umbilical cord improve septic shock in pigs
- PMID: 30091119
- PMCID: PMC6082751
- DOI: 10.1186/s40635-018-0194-1
Clinical-grade mesenchymal stem cells derived from umbilical cord improve septic shock in pigs
Abstract
Background: Septic shock is the leading cause of death in intensive care units. The pathophysiological complexity of this syndrome contributes to an absence of specific treatment. Several preclinical studies in murine models of septic shock have shown improvements to organ injury and survival after administration of mesenchymal stem cells (MSCs). To better mimic a clinical approach in humans, we investigated the impact of randomized controlled double-blind administration of clinical-grade umbilical cord-derived MSCs to a relevant pig model of septic shock.
Methods: Septic shock was induced by fecal peritonitis in 12 male domestic pigs. Animals were resuscitated by an experienced intensivist including fluid administration and vasopressors. Four hours after the induction of peritonitis, pigs were randomized to receive intravenous injection of thawed umbilical cord-derived MSCs (UCMSC) (1 × 106 UCMSCs/kg diluted in 75 mL hydroxyethyl starch (HES), (n = 6) or placebo (HES alone, n = 6). Researchers were double-blinded to the treatment administered. Hemodynamic parameters were continuously recorded. Gas exchange, acid-base status, organ function, and plasma cytokine concentrations were assessed at regular intervals until 24 h after the onset of peritonitis when animals were sacrificed under anesthesia.
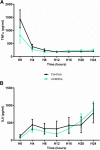
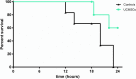
Results: Peritonitis induced profound hypotension, hyperlactatemia, and multiple organ failure. These disorders were significantly attenuated when animals were treated with UCMSCs. In particular, cardiovascular failure was attenuated, as attested by a better mean arterial pressure and reduced lactatemia, despite lower norepinephrine requirements. As such, UCMSCs improved survival in this very severe model (60% survival vs. 0% at 24 h).
Conclusion: UCMSCs administration is beneficial in this pig model of polymicrobial septic shock.
Keywords: Clinical-grade; Mesenchymal stem cells; Septic shock; Umbilical cord.
Conflict of interest statement
Umbilical cords were obtained from new mothers after they had signed an informed consent form in compliance with French national legislation relating to human sample collection, manipulation, and personal data protection. The collection protocol was approved by Nancy Hospital’s ethics committee and the French ministry for research (No. DC-2014-2114).
Experiments were performed in line with the National Institute of Health guidelines on the Use of Laboratory Animals and were approved by the University Animal Care Committee (Comité d’Éthique Lorrain en Matière d’Expérimentation Animale (CELMEA-CE2A-66) authorization no. APAFIS5674-201606141602993).
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
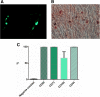
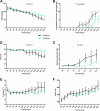
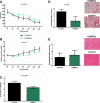
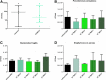
References
-
- Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:775. doi: 10.1001/jama.2016.0289. - DOI - PMC - PubMed
-
- van Vught LA, Wiewel MA, Hoogendijk AJ, Frencken JF, Scicluna BP, Klein Klouwenberg PMC et al (2017) The host response in sepsis patients developing intensive care unit-acquired secondary infections. Am J Respir Crit Care Med. 10.1164/rccm.201606-1225OC. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

