Sodium valproate versus phenytoin monotherapy for epilepsy: an individual participant data review
- PMID: 30091458
- PMCID: PMC6513104
- DOI: 10.1002/14651858.CD001769.pub4
Sodium valproate versus phenytoin monotherapy for epilepsy: an individual participant data review
Abstract
Background: Epilepsy is a common neurological condition in which abnormal electrical discharges from the brain cause recurrent unprovoked seizures. It is believed that with effective drug treatment up to 70% of individuals with active epilepsy have the potential to become seizure-free, and to go into long-term remission shortly after starting drug therapy with a single antiepileptic drug in monotherapy.Worldwide, sodium valproate and phenytoin are commonly used antiepileptic drugs for monotherapy treatment. It is generally believed that phenytoin is more effective for focal onset seizures, and that sodium pvalproate is more effective for generalised onset tonic-clonic seizures (with or without other generalised seizure types). This review is one in a series of Cochrane Reviews investigating pair-wise monotherapy comparisons. This is the latest updated version of the review first published in 2001, and updated in 2013 and 2016.
Objectives: To review the time to treatment failure, remission and first seizure of sodium valproate compared to phenytoin when used as monotherapy in people with focal onset seizures or generalised tonic-clonic seizures (with or without other generalised seizure types).
Search methods: We searched the Cochrane Epilepsy Group's Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform ICTRP on 19 February 2018. We handsearched relevant journals, contacted pharmaceutical companies, original trial investigators and experts in the field.
Selection criteria: Randomised controlled trials (RCTs) comparing monotherapy with either sodium valproate or phenytoin in children or adults with focal onset seizures or generalised onset tonic-clonic seizures DATA COLLECTION AND ANALYSIS: This was an individual participant data (IPD) review. Our primary outcome was time to treatment failure and our secondary outcomes were time to first seizure post-randomisation, time to six-month, and 12-month remission, and incidence of adverse events. We used Cox proportional hazards regression models to obtain trial-specific estimates of hazard ratios (HRs) with 95% confidence intervals (CIs), using the generic inverse variance method to obtain the overall pooled HR and 95% CI.
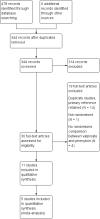
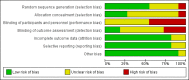
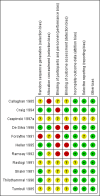
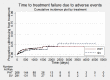
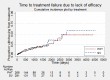
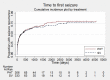
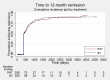
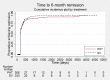
Main results: We included 11 trials in this review and IPD were available for 669 individuals out of 1119 eligible individuals from five out of 11 trials, 60% of the potential data. Results apply to focal onset seizures (simple, complex and secondary generalised tonic-clonic seizures), and generalised tonic-clonic seizures, but not other generalised seizure types (absence or myoclonus seizure types). For remission outcomes, a HR of less than 1 indicates an advantage for phenytoin, and for first seizure and treatment failure outcomes a HR of less than 1 indicates an advantage for sodium valproate.The main overall results were: time to treatment failure for any reason related to treatment (pooled HR adjusted for seizure type 0.88, 95% CI 0.61 to 1.27; 5 studies; 528 participants; moderate-quality evidence), time to treatment failure due to adverse events (pooled HR adjusted for seizure type 0.77, 95% CI 0.44 to 1.37; 4 studies; 418 participants; moderate-quality evidence), time to treatment failure due to lack of efficacy (pooled HR for all participants 1.16 (95% CI 0.71 to 1.89; 5 studies; 451 participants; moderate-quality evidence). These results suggest that treatment failure for any reason related to treatment and treatment failure due to adverse events may occur earlier on phenytoin compared to sodium valproate, while treatment failure due to lack of efficacy may occur earlier on sodium valproate than phenytoin; however none of these results were statistically significant.Results for time to first seizure (pooled HR adjusted for seizure type 1.08, 95% CI 0.88 to 1.33; 5 studies; 639 participants; low-quality evidence) suggest that first seizure recurrence may occur slightly earlier on sodium valproate compared to phenytoin. There were no clear differences between drugs in terms of time to 12-month remission (pooled HR adjusted for seizure type 1.02, 95% CI 0.81 to 1.28; 4 studies; 514 participants; moderate-quality evidence) and time to six-month remission (pooled HR adjusted for seizure type 1.05, 95% CI 0.86 to 1.27; 5 studies; 639 participants; moderate-quality evidence).Limited information was available regarding adverse events in the trials and we could not make comparisons between the rates of adverse events on sodium valproate and phenytoin. Some adverse events reported with both drugs were drowsiness, rash, dizziness, nausea and gastrointestinal problems. Weight gain was also reported with sodium valproate and gingival hypertrophy/hyperplasia was reported on phenytoin.The methodological quality of the included trials was generally good, however four out of the five trials providing IPD for analysis were of an open-label design, therefore all results were at risk of detection bias. There was also evidence that misclassification of seizure type may have confounded the results of this review, particularly for the outcome 'time to first seizure' and heterogeneity was present in analysis of treatment failure outcomes which could not be explained by subgroup analysis by epilepsy type or by sensitivity analysis for misclassification of seizure type. Therefore, for treatment failure outcomes we judged the quality of the evidence to be moderate to low, for 'time to first seizure' we judged the quality of the evidence to be low, and for remission outcomes we judged the quality of the evidence to be moderate.
Authors' conclusions: We have not found evidence that a significant difference exists between valproate and phenytoin for any of the outcomes examined in this review. However detection bias, classification bias and heterogeneity may have impacted on the results of this review. We did not find any outright evidence to support or refute current treatment policies. We recommend that future trials be designed to the highest quality possible with consideration of masking, choice of population, classification of seizure type, duration of follow-up, choice of outcomes and analysis, and presentation of results.
Conflict of interest statement
SJ Nevitt has no declarations of interest.
AG Marson: A consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to University of Liverpool. Professor Tony Marson is part funded by National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North West Coast (NIHR CLAHRC NWC).
J Weston has no declarations of interest.
C Tudur Smith has no declarations of interest.
Figures







Update of
-
Phenytoin versus valproate monotherapy for partial onset seizures and generalised onset tonic-clonic seizures: an individual participant data review.Cochrane Database Syst Rev. 2016 Apr 28;4(4):CD001769. doi: 10.1002/14651858.CD001769.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2018 Aug 09;8:CD001769. doi: 10.1002/14651858.CD001769.pub4. PMID: 27123830 Free PMC article. Updated.
References
References to studies included in this review
Callaghan 1985 {published data only}
Craig 1994 {published data only}
-
- Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single‐blind randomized comparative study. Epilepsia 1994;35(2):381‐90. - PubMed
Czapinski 1997a {unpublished data only}
-
- Czapinski P, Terczynski A, Czapinska E. Randomised 36‐month comparative study of valproic acid, phenytoin, phenobarbital and carbamazepine efficacy in patients with newly diagnosed epilepsy with partial complex seizures. Epilepsia 1997;38(Suppl 3):42.
De Silva 1996 {published data only}
-
- Silva M, MacArdle B, McGowan M, Hughes E, Stewart J, Neville BGR, et al. Randomised comparative monotherapy trial of phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly diagnosed childhood epilepsy. Lancet 1996;347:709‐13. - PubMed
Forsythe 1991 {published data only}
-
- Forsythe I, Butler R, Berg I, McGuire R. Cognitive impairment in new cases of epilepsy randomly assigned to carbamazepine, phenytoin and sodium valproate. Developmental Medicine and Child Neurology 1991;33:524‐34. - PubMed
Heller 1995 {published data only}
-
- Heller AJ, Chesterman P, Elwes RD, Crawford P, Chadwick DW, Johnson AL, et al. Phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly diagnosed adult epilepsy: a randomised comparative monotherapy trial. Journal of Neurology, Neurosurgery, and Psychiatry 1995;58:44‐50. - PMC - PubMed
Ramsay 1992 {published data only}
-
- Ramsay RE, Wilder BJ, Murphy JV, Holmes GL, Uthman B, Slater J, et al. Efficacy and safety of valproic acid versus phenytoin as sole therapy for newly diagnosed primary generalized tonic‐clonic seizures. Journal of Epilepsy 1992;5(1):55‐60.
Rastogi 1991 {published data only}
-
- Rastogi P, Mehrotra TN, Agarwala RK, Singh VS. Comparison of sodium valproate and phenytoin as single drug treatment in generalised and partial epilepsy. Journal of the Association of Physicians of India 1991;39(8):606‐8. - PubMed
Shakir 1981 {published data only}
-
- Shakir RA, Johnson RH, Lambie DG, Melville ID, Nanda RN. Comparison of sodium valproate and phenytoin as single drug treatment in epilepsy. Epilepsia 1981;22:27‐33. - PubMed
Thilothammal 1996 {published data only}
-
- Thilothammal N, Banu K, Ratnam RS. Comparison of phenobarbitone, phenytoin with sodium valproate: randomized, double‐blind study. Indian Pediatrics 1996;33:549‐55. - PubMed
References to studies excluded from this review
Berg 1993 {published data only}
-
- Berg I, Butler A, Ellis M, Foster J. Psychiatric aspects of epilepsy in childhood treated with carbamazepine, phenytoin or sodium valproate: a random trial. Developmental Medicine and Child Neurology 1993;35:149‐57. - PubMed
Callaghan 1981 {published data only}
-
- Callaghan N, O'Neill B, Kenny RA. A comparison between carbamazepine, phenytoin and sodium valproate as single drug treatment in epilepsy [abstract]. Irish Journal of Medical Science 1981;150(6):194.
Callaghan 1983 {published data only}
-
- Callaghan N, Kenny RA, O'Neill B, Crowley M, Goggin T. A comparative study between carbamazepine, phenytoin and sodium valproate as monotherapy in previously untreated and recently diagnosed patients with epilepsy: a preliminary communication. British Journal of Clinical Practice 1983;27:7‐9.
Callaghan 1984 {published data only}
-
- Callaghan N, Kenny RA, O'Neill B, Crowley M, Goggin T. A comparative study of carbamazepine, sodium valproate and phenytoin as monotherapy in epilepsy: an updated report [abstract]. Irish Journal of Medical Science 1984;153(4):154.
Craig 1993 {published data only}
-
- Craig IR, Tallis RC. The impact of sodium valproate and phenytoin on cognitive function in elderly patients: results of a single‐blind comparative study. Age and Ageing 1993;22(Suppl 2):10. - PubMed
Czapinski 1997b {published data only}
-
- Czapinski P, Terczynski A, Czapinska E. Comparative study of valproic acid (VPA), phenytoin (PHT), phenobarbital (PB) and carbamazepine (CBZ) in patients with newly diagnosed epilepsy with partial complex seizures [abstract]. Journal of Neurology 1997;244(Suppl 3):S95‐6.
Czapinski 1997c {published data only}
-
- Czapinski P, Terczynski A, Czapinska E. Randomized 36‐month comparative study of valproic acid (VPA), phenytoin (PHT), phenobarbital (PB) and carbamazepine (CBZ) efficacy in patients with newly diagnosed epilepsy with partial complex seizures. Journal of the Neurological Sciences 1997;150(Suppl):S162‐3.
Goggin 1984 {published data only}
-
- Goggin T. A re‐appraisal by control and seizure type of serum levels in previously untreated patients taking part in a prospective study comparing sodium valproate, phenytoin and carbamazepine as mono‐therapy in epilepsy [abstract]. Irish Journal of Medical Science 1984;153(4):154.
Goggin 1986 {published data only}
-
- Goggin T, Casey C, Callaghan N. Serum levels of sodium valproate, phenytoin and carbamazepine and seizure control in epilepsy. Irish Medical Journal 1986;79(6):150‐6. - PubMed
Jannuzzi 2000 {published data only}
-
- Jannuzzi G, Cian P, Fattore C, Gatti G, Bartoli A, Monaco F, et al. A multicenter randomized controlled trial on the clinical impact of therapeutic drug monitoring in patients with newly diagnosed epilepsy. The Italian TDM Study Group in Epilepsy. Epilepsia 2000;41(2):222‐30. - PubMed
Kaminow 2003 {published data only}
-
- Kaminow L, Schimschock JR, Hammer AE, Vuong A. Lamotrigine monotherapy compared with carbamazepine, phenytoin, or valproate monotherapy in patients with epilepsy. Epilepsy and Behaviour 2003;4(6):659‐66. - PubMed
Sabers 1995 {published data only}
-
- Sabers A, Møller A, Dam M, Smed A, Arlien‐Søborg P, Buchman J, et al. Cognitive function and anticonvulsant therapy: effect of monotherapy in epilepsy. Acta Neurologica Scandinavica 1995;92(1):19‐27. - PubMed
Schmidt 2007 {published data only}
-
- Schmidt D. How reliable is early treatment response in predicting long‐term seizure outcome?. Epilepsy and Behaviour 2007;10(4):588‐94. - PubMed
Shakir 1980 {published data only}
-
- Shakir RA. Sodium valproate, phenytoin and carbamazepine as sole anticonvulsants. Royal Society of Medicine International Congress and Symposium 1980;30:7‐16.
Tallis 1994a {published data only}
-
- Tallis R, Craig I, Easter D. Multicentre comparative trial of sodium valproate and phenytoin in elderly patients with newly diagnosed epilepsy. Age and Ageing 1994;23:S5.
Tallis 1994b {published data only}
-
- Tallis R, Easter D. Multicenter comparative trial of valproate and phenytoin. Epilepsia 1994;35(Suppl 7):62. - PubMed
Turnbull 1982 {published data only}
Wilder 1983 {published data only}
-
- Wilder BJ, Ramsay RE, Murphy JV, Karas BJ, Marquardt K, Hammond EJ. Comparison of valproic acid and phenytoin in newly diagnosed tonic‐clonic seizures. Neurology 1983;33(11):1474‐6. - PubMed
Zeng 2010 {published and unpublished data}
-
- Zeng K, Wang X, Xi Z, Yan Y. Adverse effects of carbamazepine, phenytoin, valproate and lamotrigine monotherapy in epileptic adult Chinese patients. Clinical Neurology and Neurosurgery 2010;112:291‐5. - PubMed
Additional references
Annegers 1999
-
- Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia 1999;40(4):502‐6. - PubMed
Bodensteiner 1988
-
- Bodensteiner JB, Brownsworth RD, Knapik JR, Kanter MC, Cowan LD, Leviton A. Interobserver variability in the ILAE classification of seizures in childhood. Epilepsia 1988;29(2):123‐8. - PubMed
Bourgeois 1987
-
- Bourgeois B, Beaumanoir A, Blajev B, Cruz ND, Despland PA, Egli M, et al. Monotherapy with valproate in primary generalized epilepsies. Epilepsia 1987;28(Suppl 2):S8‐11. - PubMed
Brasfield 1999
-
- Brasfield KH. Pilot study of divalproex sodium valproate versus valproic acid: drug acquisition costs versus all related costs. Current Therapeutic Research 1999;60(3):138‐44.
Bromley 2013
-
- Bromley RL, Mawer GE, Briggs M, Cheyne C, Clayton‐Smith J, García‐Fiñana M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. Journal of Neurology, Neurosurgery and Psychiatry 2013;84(6):637‐43. [DOI: 10.1136/jnnp-2012-304270] - DOI - PMC - PubMed
Bromley 2014
Canger 1999
-
- Canger R, Battino D, Canevini MP, Fumarola C, Guidolin L, Vignoli A, et al. Malformations in offspring of women with epilepsy: A prospective study. Epilepsia 1999;40(9):1231‐6. - PubMed
Carl 1992
-
- Carl GF, Smith ML. Phenytoin‐folate interactions: differing effects of the sodium salt and the free acid of phenytoin. Epilepsia 1992;33(2):372‐5. - PubMed
Chadwick 1994
-
- Chadwick DW. Valproate in the treatment of partial epilepsies. Epilepsia 1994;35(5):S96‐8. - PubMed
Cockerell 1995
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet 1995;346(8968):140‐4. - PubMed
Commission 1981
-
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia 1981;22(4):489‐501. - PubMed
Commission 1989
-
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30(4):389‐99. - PubMed
Cranor 1997
-
- Cranor CW, Sawyer WT, Carson SW, Early JJ. Clinical and economic impact of replacing divalproex sodium with valproic acid. American Journal of Health‐System Pharmacy 1997;54:1716‐22. - PubMed
Delgado‐Escueta 1984
-
- Delgado‐Escueta AV, Enrile‐Bascal F. Juvenile myoclonic epilepsy of Janz. Neurology 1984;34(3):285‐94. - PubMed
Dinesen 1984
-
- Dinesen H, Gram L, Andersen T, Dam M. Weight gain during treatment with valproate. Acta Neurologica Scandinavica 1984;69:65‐9. - PubMed
Easter 1997
-
- Easter D, O'Bryan‐Tear CG, Verity C. Weight gain with valproate or carbamazepine‐a reappraisal. Seizure 1997;6(2):121‐5. - PubMed
Egger 1981
Gladstone 1992
-
- Gladstone DJ, Bologa M, Maguire C, Pastuszak A, Koren G. Course of pregnancy and fetal outcome following maternal exposure to carbamazepine and phenytoin: a prospective study. Reproductive Toxicology 1992;6(3):257‐61. - PubMed
Hauser 1993
-
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota 1935 ‐ 1984. Epilepsia 1993;34:453‐68. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Hirtz 2007
-
- Hirtz D, Thurman DJ, Gwinn‐Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders?. Neurology 2007;68:326‐37. - PubMed
ILAE 1998
-
- ILAE Commission on Antiepileptic Drugs. Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia 1998;39(7):799‐803. - PubMed
ILAE 2006
-
- Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Chadwick D, Guerreiro C, et al. ILAE treatment guidelines: Evidence based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2006;47(7):1094‐120. - PubMed
Jeavons 1977
-
- Jeavons PM. Choice of drug therapy in epilepsy. Practitioner 1977;219:542‐56. - PubMed
Jones 1996
Juul Jenson 1983
-
- Juul‐Jenson P, Foldspang A. Natural history of epileptic seizures. Epilepsia 1983;24:297‐312. - PubMed
Kirkham 2010
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. - PubMed
Kwan 2000
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine 2000;342:314‐9. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liporace 1994
-
- Liporace JD, Sperling MR, Dichter MA. Absence seizures and carbamazepine in adults. Epilepsia 1994;35(5):1026‐8. - PubMed
MacDonald 2000
-
- MacDonald BK, Johnson AL, Goodridge DM, Cockerell OC, Sander JWA, Shorvon SD. Factors predicting prognosis of epilepsy after presentation with seizures. Annals of Neurology 2000;48:833‐41. - PubMed
Malafosse 1994
-
- Malafosse A, Genton P, Hirsch E, Marescaux C, Broglin D, Bernasconi R, editor(s), et al. Idiopathic Generalised Epilepsies: Clinical, Experimental and Genetic Aspects. Eastleigh: John Libbey and Company, 1994. [0861964365]
Marson 2000
Mattson 1985
-
- Mattson RH, Cramer JA, Collins JF, Smith DB, Delgado‐Escueta AV, Browne TR, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic‐clonic seizures. New England Journal of Medicine 1985;313(3):145‐51. - PubMed
Meador 2008
Morrow 2006
Murray 1994
-
- Murray CJL, Lopez AD, World Health Organization. Global comparative assessments in the health sector: disease burden, expenditures and intervention packages. apps.who.int/iris/handle/10665/41177 (accessed 01 08 2018).
Nevitt 2017a
Nevitt 2017b
Nevitt 2018
Ngugi 2010
NICE 2012
-
- National Institute for Health and Care Excellence. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. www.nice.org.uk/guidance/cg137 (accessed 01 08 2018).
Nolan 2013b
Nolan 2013c
Nolan 2013d
-
- Nolan SJ, Sutton L, Marson A, Tudur Smith C. Consistency of outcome and statistical reporting of time‐to‐event data: the impact on Cochrane Reviews and meta‐analyses in epilepsy. 21st Cochrane Colloquium: Better Knowledge for Better Health, Quebec City. 2013:114‐5.
Nolan 2016b
Nolan 2016c
Novak 1999
-
- Novak GP, Maytal J, Alshansky A, Eviatar L, Sy‐Kho R, Siddique Q. Risk of excessive weight gain in epileptic children treated with valproate. Journal of Child Neurology 1999;14(8):490‐5. - PubMed
Nulman 1997
-
- Nulman I, Scolnik D, Chitayat D, Farkas LD, Koren G. Findings in children exposed in utero to phenytoin and carbamazepine monotherapy: independent effects of epilepsy and medications. American Journal of Medical Genetics 1997;68(1):18‐24. - PubMed
Olafsson 2005
-
- Olafsson E, Ludvigsson P, Gudmundsson G, Hesdorfer D, Kjartansson O, Hauser WA. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurology 2005;4:627‐34. - PubMed
Ornoy 2009
-
- Ornoy A. Valproic acid in pregnancy: How much are we endangering the embryo and fetus?. Reproductive Toxicology 2009;28(1):1‐10. - PubMed
Ottman 1993
Parmar 1998
-
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Penry 1989
-
- Penry JK, Dean JC, Riela AR. Juvenile myoclonic epilepsy: long term response to therapy. Epilepsia 1989;30(Suppl 4):S19‐23. - PubMed
Sander 1996
Sander 2004
-
- Sander JW. The use of anti‐epileptic drugs ‐ principles and practice. Epilepsia 2004;45(6):28‐34. - PubMed
Scheffer 2017
Scheinfeld 2003
-
- Scheinfeld N. Phenytoin in cutaneous medicine: Its uses, mechanisms and side effects. Dermatology Online Journal 2003;9(3):6. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html. GRADE Working Group, 2013.
Stata 2015 [Computer program]
-
- StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP, 2015.
Tomson 2011
-
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Dose‐dependent risk of malformation with antiepileptic drugs: An analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurology 2011;10(7):609‐17. - PubMed
Troupin 1975
-
- Troupin AS, Ojemann LM. Paradoxical intoxication ‐ a complication of anticonvulsant administration. Epilepsia 1975;16:753‐8. - PubMed
Tudur Smith 2007
Vallarta 1974
-
- Vallarta JM, Bell DB, Reichert A. Progressive encephalopathy due to chronic hydantoin intoxication. American Journal of Diseases of Children 1974;128:27‐34. - PubMed
Wallace 1997
-
- Wallace H, Shorvon SD, Hopkins A, O'Donoghue M. National Society of Epilepsy Guidelines. London: Royal College of Physicians 1997.
Weston 2017
Wilder 1983a
-
- Wilder BJ, Karas BJ, Penry JK, Asconape J. Gastrointestinal tolerance of divalproex sodium. Neurology 1983;33:808‐11. - PubMed
Wilder 1995
-
- Wilder BJ. Phenytoin: clinical use. Antiepileptic Drugs. New York: Raven Press, 1995:339‐44.
Williamson 2000
-
- Williamson PR, Marson AG, Tudur C, Hutton JL, Chadwick DW. Individual patient data meta‐analysis of randomized anti‐epileptic drug monotherapy trials. Journal of Evaluation in Clinical Practice 2000;6(2):205‐14. - PubMed
Williamson 2002
-
- Williamson PR, Tudur Smith C, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(11):3337‐51. - PubMed
References to other published versions of this review
Nolan 2013a
Nolan 2016a
-
- Nolan SJ, Marson AG, Weston J, Tudur Smith C. Phenytoin versus valproate monotherapy for partial onset seizures and generalised onset tonic‐clonic seizures: an individual participant data review. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD001769.pub3] - DOI - PMC - PubMed
Tudur 1999
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

