WDR62 mediates TNFα-dependent JNK activation via TRAF2-MLK3 axis
- PMID: 30091641
- PMCID: PMC6233063
- DOI: 10.1091/mbc.E17-08-0504
WDR62 mediates TNFα-dependent JNK activation via TRAF2-MLK3 axis
Abstract
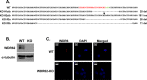
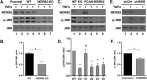
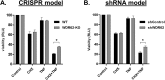
The mitogen-activated protein kinases (MAPKs) regulate a variety of cellular processes. The three main MAPK cascades are the extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinase (JNK), and p38 kinases. A typical MAPK cascade is composed of MAP3K-MAP2K-MAPK kinases that are held by scaffold proteins. Scaffolds function to assemble the protein tier and contribute to the specificity and efficacy of signal transmission. WD repeat domain 62 (WDR62) is a JNK scaffold protein, interacting with JNK, MKK7, and several MAP3Ks. The loss of WDR62 in human leads to microcephaly and pachygyria. Yet the role of WDR62 in cellular function is not fully studied. We used the CRISPR/Cas9 and short hairpin RNA approaches to establish a human breast cancer cell line MDA-MB-231 with WDR62 loss of function and studied the consequence to JNK signaling. In growing cells, WDR62 is responsible for the basal expression of c-Jun. In stressed cells, WDR62 specifically mediates TNFα-dependent JNK activation through the association with both the adaptor protein, TNF receptor-associated factor 2 (TRAF2), and the MAP3K protein, mixed lineage kinase 3. TNFα-dependent JNK activation is mediated by WDR62 in HCT116 and HeLa cell lines as well. MDA-MB-231 WDR62-knockout cells display increased resistance to TNFα-induced cell death. Collectively, WDR62 coordinates the TNFα receptor signaling pathway to JNK activation through association with multiple kinases and the adaptor protein TRAF2.
Figures





References
-
- Aggarwal BB. (2003). Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol , 745–756. - PubMed
-
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. (2000). The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem , 9047–9054. - PubMed
-
- Balkwill F. (2009). Tumour necrosis factor and cancer. Nat Rev Cancer , 361–371. - PubMed
-
- Batard P, Jordan M, Wurm F. (2001). Transfer of high copy number plasmid into mammalian cells by calcium phosphate transfection. Gene , 61–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

