Flexible resonance in prefrontal networks with strong feedback inhibition
- PMID: 30091975
- PMCID: PMC6103521
- DOI: 10.1371/journal.pcbi.1006357
Flexible resonance in prefrontal networks with strong feedback inhibition
Abstract
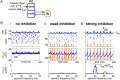
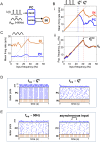
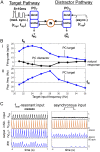
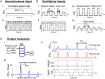
Oscillations are ubiquitous features of brain dynamics that undergo task-related changes in synchrony, power, and frequency. The impact of those changes on target networks is poorly understood. In this work, we used a biophysically detailed model of prefrontal cortex (PFC) to explore the effects of varying the spike rate, synchrony, and waveform of strong oscillatory inputs on the behavior of cortical networks driven by them. Interacting populations of excitatory and inhibitory neurons with strong feedback inhibition are inhibition-based network oscillators that exhibit resonance (i.e., larger responses to preferred input frequencies). We quantified network responses in terms of mean firing rates and the population frequency of network oscillation; and characterized their behavior in terms of the natural response to asynchronous input and the resonant response to oscillatory inputs. We show that strong feedback inhibition causes the PFC to generate internal (natural) oscillations in the beta/gamma frequency range (>15 Hz) and to maximize principal cell spiking in response to external oscillations at slightly higher frequencies. Importantly, we found that the fastest oscillation frequency that can be relayed by the network maximizes local inhibition and is equal to a frequency even higher than that which maximizes the firing rate of excitatory cells; we call this phenomenon population frequency resonance. This form of resonance is shown to determine the optimal driving frequency for suppressing responses to asynchronous activity. Lastly, we demonstrate that the natural and resonant frequencies can be tuned by changes in neuronal excitability, the duration of feedback inhibition, and dynamic properties of the input. Our results predict that PFC networks are tuned for generating and selectively responding to beta- and gamma-rhythmic signals due to the natural and resonant properties of inhibition-based oscillators. They also suggest strategies for optimizing transcranial stimulation and using oscillatory networks in neuromorphic engineering.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ (2012) Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 32: 12411–12420. 10.1523/JNEUROSCI.0421-12.2012 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

