Extended darkness induces internal turnover of glucosinolates in Arabidopsis thaliana leaves
- PMID: 30092103
- PMCID: PMC6084957
- DOI: 10.1371/journal.pone.0202153
Extended darkness induces internal turnover of glucosinolates in Arabidopsis thaliana leaves
Abstract
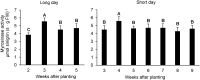
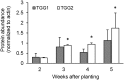
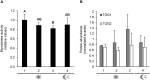
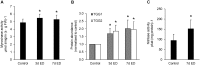
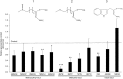
Prolonged darkness leads to carbohydrate starvation, and as a consequence plants degrade proteins and lipids to oxidize amino acids and fatty acids as alternative substrates for mitochondrial ATP production. We investigated, whether the internal breakdown of glucosinolates, a major class of sulfur-containing secondary metabolites, might be an additional component of the carbohydrate starvation response in Arabidopsis thaliana (A. thaliana). The glucosinolate content of A. thaliana leaves was strongly reduced after seven days of darkness. We also detected a significant increase in the activity of myrosinase, the enzyme catalyzing the initial step in glucosinolate breakdown, coinciding with a strong induction of the main leaf myrosinase isoforms TGG1 and TGG2. In addition, nitrilase activity was increased suggesting a turnover via nitriles and carboxylic acids. Internal degradation of glucosinolates might also be involved in diurnal or developmental adaptations of the glucosinolate profile. We observed a diurnal rhythm for myrosinase activity in two-week-old plants. Furthermore, leaf myrosinase activity and protein abundance of TGG2 varied during plant development, whereas leaf protein abundance of TGG1 remained stable indicating regulation at the transcriptional as well as post-translational level.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996;97(1):194–208. 10.1111/j.1399-3054.1996.tb00497.x - DOI
-
- Brown PD, Morra MJ. Control of Soil-Borne Plant Pests Using Glucosinolate-Containing Plants. Adv. Agron. 1997;61(C):167–231. 10.1016/S0065-2113(08)60664-1 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

