International pilot external quality assessment scheme for analysis and reporting of circulating tumour DNA
- PMID: 30092778
- PMCID: PMC6085634
- DOI: 10.1186/s12885-018-4694-x
International pilot external quality assessment scheme for analysis and reporting of circulating tumour DNA
Abstract
Background: Molecular analysis of circulating tumour DNA (ctDNA) is becoming increasingly important in clinical treatment decisions. A pilot External Quality Assessment (EQA) scheme for ctDNA analysis was organized by four European EQA providers under the umbrella organization IQN Path, in order to investigate the feasibility of delivering an EQA to assess the detection of clinically relevant variants in plasma circulating cell-free DNA (cfDNA) and to analyze reporting formats.
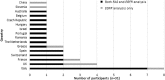
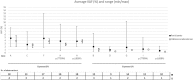
Methods: Thirty-two experienced laboratories received 5 samples for EGFR mutation analysis and/or 5 samples for KRAS and NRAS mutation analysis. Samples were artificially manufactured to contain 3 mL of human plasma with 20 ng/mL of fragmented ctDNA and variants at allelic frequencies of 1 and 5%.
Results: The scheme error rate was 20.1%. Higher error rates were observed for RAS testing when compared to EGFR analysis, for allelic frequencies of 1% compared to 5%, and for cases including 2 different variants. The reports over-interpreted wild-type results and frequently failed to comment on the amount of cfDNA extracted.
Conclusions: The pilot scheme demonstrated the feasibility of delivering a ctDNA EQA scheme and the need for such a scheme due to high error rates in detecting low frequency clinically relevant variants. Recommendations to improve reporting of cfDNA are provided.
Keywords: Colorectal cancer; EGFR; KRAS; Lung cancer; Mutation testing; NRAS; cfDNA; ctDNA.
Conflict of interest statement
Not applicable.
Not applicable.
EMCD received research grants from Pfizer and Amgen. SJP received financial support for educational programmes from Astra Zeneca. NN received fees or research funds from Roche, Qiagen, Thermofisher, Merck, and Astrazeneca. JAH owns stock in Vivactiv Ltd. ES performed lectures for Illumina, Novartis, Pfizer, BioCartis; is consultant in advisory boards for AstraZeneca, Pfizer, Novartis, BioCartis; and received financial support from Roche, Biocartis, BMS, Pfizer (all fees to the Institution). ZCD received financial support for educational programmes from Astra Zeneca, Roche and Qiagen and is a member of advisory boards for Amgen, Astra Zeneca, Pfizer, Merck Serono and Roche. All other authors have nothing to declare.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

