Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia
- PMID: 30093115
- PMCID: PMC6398932
- DOI: 10.1016/j.bbrc.2018.08.019
Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia
Abstract
Mesenchymal stromal cell (MSC) derived exosomes mediate tissue protection and regeneration in many injuries and diseases by modulating cell protein production, protecting from apoptosis, inhibiting inflammation, and increasing angiogenesis. In the present study, daily intraperitoneal injection of MSC-derived exosomes protected alveolarization and angiogenesis in a newborn rat model of bronchopulmonary dysplasia (BPD) induced by 14 days of neonatal hyperoxia exposure (85% O2). Exosome treatment during hyperoxia prevented disruption of alveolar growth, increased small blood vessel number, and inhibited right heart hypertrophy at P14, P21, and P56. In vitro, exosomes significantly increased tube-like network formation by HUVEC, in part through a VEGF mediated mechanism. In summary, daily intraperitoneal injection of exosomes increased blood vessel number and size in the lung through pro-angiogenic mechanisms. MSC-derived exosomes therefore have both anti-inflammatory and pro-angiogenic mechanism to protect the lung from hyperoxia induced lung and heart disease associated with BPD.
Keywords: Bronchopulmonary dysplasia; Chronic lung disease; Exosomes; Lung prematurity; Mesenchymal stromal cells.
Published by Elsevier Inc.
Conflict of interest statement
Conflicts of interest
The authors declare no conflicts of interest.
Figures

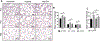
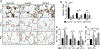

References
-
- Warner BB, Stuart LA, Papes RA, Wispe JR, Functional and pathological effects of prolonged hyperoxia in neonatal mice, Am J Physiol, 275 (1998) L110–117. - PubMed
-
- Bhandari A, McGrath-Morrow S, Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia, Seminars in perinatology, 37 (2013) 132–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

