Systemic AAV Micro-dystrophin Gene Therapy for Duchenne Muscular Dystrophy
- PMID: 30093306
- PMCID: PMC6171037
- DOI: 10.1016/j.ymthe.2018.07.011
Systemic AAV Micro-dystrophin Gene Therapy for Duchenne Muscular Dystrophy
Abstract
Duchenne muscular dystrophy (DMD) is a lethal muscle disease caused by dystrophin gene mutation. Conceptually, replacing the mutated gene with a normal one would cure the disease. However, this task has encountered significant challenges due to the enormous size of the gene and the distribution of muscle throughout the body. The former creates a hurdle for viral vector packaging and the latter begs for whole-body therapy. To address these obstacles, investigators have invented the highly abbreviated micro-dystrophin gene and developed body-wide systemic gene transfer with adeno-associated virus (AAV). Numerous microgene configurations and various AAV serotypes have been explored in animal models in many laboratories. Preclinical data suggests that intravascular AAV micro-dystrophin delivery can significantly ameliorate muscle pathology, enhance muscle force, and attenuate dystrophic cardiomyopathy in animals. Against this backdrop, several clinical trials have been initiated to test the safety and tolerability of this promising therapy in DMD patients. While these trials are not powered to reach a conclusion on clinical efficacy, findings will inform the field on the prospects of body-wide DMD therapy with a synthetic micro-dystrophin AAV vector. This review discusses the history, current status, and future directions of systemic AAV micro-dystrophin therapy.
Keywords: AAV; BMD; Becker muscular dystrophy; DMD; Duchenne muscular dystrophy; adeno-associated virus; clinical trial; dystrophin; micro-dystrophin; systemic gene therapy.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




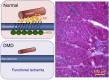
References
-
- Drouin E., Péréon Y. Duchenne or Meryon muscular dystrophy? Mol. Genet. Metab. 2014;113:241–242. - PubMed
-
- Mendell J.R., Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve. 2013;48:21–26. - PubMed
-
- Bushby K., Finkel R., Birnkrant D.J., Case L.E., Clemens P.R., Cripe L., Kaul A., Kinnett K., McDonald C., Pandya S., DMD Care Considerations Working Group Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. - PubMed
-
- Mendell J.R., Province M.A., Moxley R.T., 3rd, Griggs R.C., Brooke M.H., Fenichel G.M., Miller J.P., Kaiser K.K., King W., Robison J. Clinical investigation of Duchenne muscular dystrophy. A methodology for therapeutic trials based on natural history controls. Arch. Neurol. 1987;44:808–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

