Chronic d-serine supplementation impairs insulin secretion
- PMID: 30093356
- PMCID: PMC6157639
- DOI: 10.1016/j.molmet.2018.07.002
Chronic d-serine supplementation impairs insulin secretion
Abstract
Objective: The metabolic role of d-serine, a non-proteinogenic NMDA receptor co-agonist, is poorly understood. Conversely, inhibition of pancreatic NMDA receptors as well as loss of the d-serine producing enzyme serine racemase have been shown to modulate insulin secretion. Thus, we aim to study the impact of chronic and acute d-serine supplementation on insulin secretion and other parameters of glucose homeostasis.
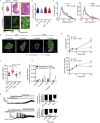
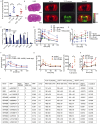
Methods: We apply MALDI FT-ICR mass spectrometry imaging, NMR based metabolomics, 16s rRNA gene sequencing of gut microbiota in combination with a detailed physiological characterization to unravel the metabolic action of d-serine in mice acutely and chronically treated with 1% d-serine in drinking water in combination with either chow or high fat diet feeding. Moreover, we identify SNPs in SRR, the enzyme converting L-to d-serine and two subunits of the NMDA receptor to associate with insulin secretion in humans, based on the analysis of 2760 non-diabetic Caucasian individuals.
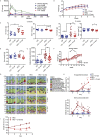
Results: We show that chronic elevation of d-serine results in reduced high fat diet intake. In addition, d-serine leads to diet-independent hyperglycemia due to blunted insulin secretion from pancreatic beta cells. Inhibition of alpha 2-adrenergic receptors rapidly restores glycemia and glucose tolerance in d-serine supplemented mice. Moreover, we show that single nucleotide polymorphisms (SNPs) in SRR as well as in individual NMDAR subunits are associated with insulin secretion in humans.
Conclusion: Thus, we identify a novel role of d-serine in regulating systemic glucose metabolism through modulating insulin secretion.
Keywords: Diabetes; Insulin secretion; Obesity; d-serine.
Copyright © 2018 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures

References
-
- Pallares-Mendez R., Aguilar-Salinas C.A., Cruz-Bautista I., Del Bosque-Plata L. Metabolomics in diabetes, a review. Annals of Medicine. 2016;48:89–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

