Structural insight into proline cis/ trans isomerization of unfolded proteins catalyzed by the trigger factor chaperone
- PMID: 30093407
- PMCID: PMC6166725
- DOI: 10.1074/jbc.RA118.003579
Structural insight into proline cis/ trans isomerization of unfolded proteins catalyzed by the trigger factor chaperone
Abstract
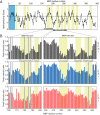
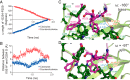
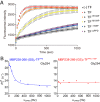
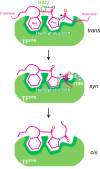
Molecular chaperones often possess functional modules that are specialized in assisting the formation of specific structural elements, such as a disulfide bridges and peptidyl-prolyl bonds in cis form, in the client protein. A ribosome-associated molecular chaperone trigger factor (TF), which has a peptidyl-prolyl cis/trans isomerase (PPIase) domain, acts as a highly efficient catalyst in the folding process limited by peptidyl-prolyl isomerization. Herein we report a study on the mechanism through which TF recognizes the proline residue in the unfolded client protein during the cis/trans isomerization process. The solution structure of TF in complex with the client protein showed that TF recognizes the proline-aromatic motif located in the hydrophobic stretch of the unfolded client protein through its conserved hydrophobic cleft, which suggests that TF preferentially accelerates the isomerization of the peptidyl-prolyl bond that is eventually folded into the core of the protein in its native fold. Molecular dynamics simulation revealed that TF exploits the backbone amide group of Ile195 to form an intermolecular hydrogen bond with the carbonyl oxygen of the amino acid residue preceding the proline residue at the transition state, which presumably stabilizes the transition state and thus accelerates the isomerization. The importance of such intermolecular hydrogen-bond formation during the catalysis was further corroborated by the activity assay and NMR relaxation analysis.
Keywords: hydrophobic interaction; molecular chaperone; molecular dynamics; nuclear magnetic resonance (NMR); peptidyl-prolyl isomerase domain; prolyl isomerase; protein folding; structure-function; trigger factor.
© 2018 Kawagoe et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

