Humanized UGT1 Mice, Regulation of UGT1A1, and the Role of the Intestinal Tract in Neonatal Hyperbilirubinemia and Breast Milk-Induced Jaundice
- PMID: 30093417
- PMCID: PMC6199628
- DOI: 10.1124/dmd.118.083212
Humanized UGT1 Mice, Regulation of UGT1A1, and the Role of the Intestinal Tract in Neonatal Hyperbilirubinemia and Breast Milk-Induced Jaundice
Abstract
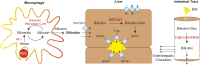
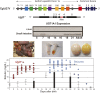
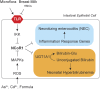
Neonatal hyperbilirubinemia and the onset of bilirubin encephalopathy and kernicterus result in part from delayed expression of UDP-glucuronosyltransferase 1A1 (UGT1A1) and the ability to metabolize bilirubin. It is generally believed that acute neonatal forms of hyperbilirubinemia develop due to an inability of hepatic UGT1A1 to metabolize efficiently bilirubin for clearance through the hepatobiliary tract. Newly developed mouse models designed to study bilirubin metabolism have led to new insight into the role of the intestinal tract in controlling neonatal hyperbilirubinemia. Humanization of mice with the UGT1 locus (hUGT1 mice) and the UGT1A1 gene provide a unique tool to study the onset of hyperbilirubinemia since the human UGT1A1 gene is developmentally regulated during the neonatal period in hUGT1 mice. A new mechanism outlying developmental expression of intestinal UGT1A1 is presented and its implications in the control of neonatal hyperbilirubinemia discussed. New findings linking breast milk protection against necrotizing enterocolitis and intestinal control of UGT1A1 may help explain the contribution of breast milk toward the development of neonatal hyperbilirubinemia. Our findings outline a new model that includes an active intestinal ROS /IκB kinase/nuclear receptor corepressor 1 loop that can be applied to an understanding of breast milk-induced jaundice.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-κB leads to hyperbilirubinemia.Gastroenterology. 2012 Jan;142(1):109-118.e2. doi: 10.1053/j.gastro.2011.09.045. Epub 2011 Oct 6. Gastroenterology. 2012. PMID: 21983082 Free PMC article.
-
Intestinal NCoR1, a regulator of epithelial cell maturation, controls neonatal hyperbilirubinemia.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):E1432-E1440. doi: 10.1073/pnas.1700232114. Epub 2017 Feb 6. Proc Natl Acad Sci U S A. 2017. PMID: 28167773 Free PMC article.
-
Cadmium and arsenic override NF-κB developmental regulation of the intestinal UGT1A1 gene and control of hyperbilirubinemia.Biochem Pharmacol. 2016 Jun 15;110-111:37-46. doi: 10.1016/j.bcp.2016.04.003. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27060662 Free PMC article.
-
Role of extrahepatic UDP-glucuronosyltransferase 1A1: Advances in understanding breast milk-induced neonatal hyperbilirubinemia.Toxicol Appl Pharmacol. 2015 Nov 15;289(1):124-32. doi: 10.1016/j.taap.2015.08.018. Epub 2015 Sep 2. Toxicol Appl Pharmacol. 2015. PMID: 26342858 Free PMC article. Review.
-
Developmental, Genetic, Dietary, and Xenobiotic Influences on Neonatal Hyperbilirubinemia.Mol Pharmacol. 2017 May;91(5):545-553. doi: 10.1124/mol.116.107524. Epub 2017 Mar 10. Mol Pharmacol. 2017. PMID: 28283555 Free PMC article. Review.
Cited by
-
Experimental models assessing bilirubin neurotoxicity.Pediatr Res. 2020 Jan;87(1):17-25. doi: 10.1038/s41390-019-0570-x. Epub 2019 Sep 7. Pediatr Res. 2020. PMID: 31493769 Review.
-
Regulation of Intestinal UDP-Glucuronosyltransferase 1A1 by the Farnesoid X Receptor Agonist Obeticholic Acid Is Controlled by Constitutive Androstane Receptor through Intestinal Maturation.Drug Metab Dispos. 2021 Jan;49(1):12-19. doi: 10.1124/dmd.120.000240. Epub 2020 Nov 5. Drug Metab Dispos. 2021. PMID: 33154041 Free PMC article.
-
Breast Milk Constituents and the Development of Breast Milk Jaundice in Neonates: A Systematic Review.Nutrients. 2023 May 10;15(10):2261. doi: 10.3390/nu15102261. Nutrients. 2023. PMID: 37242142 Free PMC article.
-
NRF2-Independent Regulation of Intestinal Constitutive Androstane Receptor by the Pro-Oxidants Cadmium and Isothiocyanate in hUGT1 Mice.Drug Metab Dispos. 2020 Jan;48(1):25-30. doi: 10.1124/dmd.119.089508. Epub 2019 Nov 8. Drug Metab Dispos. 2020. PMID: 31704714 Free PMC article.
-
BilR is a gut microbial enzyme that reduces bilirubin to urobilinogen.Nat Microbiol. 2024 Jan;9(1):173-184. doi: 10.1038/s41564-023-01549-x. Epub 2024 Jan 3. Nat Microbiol. 2024. PMID: 38172624 Free PMC article.
References
-
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. (1999) Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274:26071–26078. - PubMed
-
- Bacq Y, Zarka O, Bréchot JF, Mariotte N, Vol S, Tichet J, Weill J. (1996) Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology 23:1030–1034. - PubMed
-
- Bhutani VK, Johnson-Hamerman L. (2015) The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Fetal Neonatal Med 20:6–13. - PubMed
-
- Bhutani VK, Zipursky A, Blencowe H, Khanna R, Sgro M, Ebbesen F, Bell J, Mori R, Slusher TM, Fahmy N, et al. (2013) Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res 74 (Suppl 1):86–100. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

