Isolation and characterisation of alveolar type II pneumocytes from adult bovine lung
- PMID: 30093682
- PMCID: PMC6085293
- DOI: 10.1038/s41598-018-30234-x
Isolation and characterisation of alveolar type II pneumocytes from adult bovine lung
Abstract
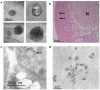
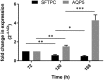
Alveolar type II (ATII) cells play a key role as part of the distal lung epithelium, including roles in the innate immune response and as self-renewing progenitors to replace alveolar type I (ATI) cells during regeneration of the alveolar epithelium. Their secretion of surfactant protein helps to maintain homeostasis in the distal lung and exert protective, antimicrobial properties. Despite the cell's crucial roles, they remain difficult to study, in part due to inefficient and expensive isolation methods, a propensity to differentiate into alveolar type I cells in culture and susceptibility to fibroblast overgrowth from primary isolations. Published methods of isolation often require specialist technology, negatively impacting the development of in vitro models of disease, including bovine tuberculosis (BTB), a serious re-emerging disease in both animals and humans worldwide. We present here a simple and cost-effective method that may be utilised in the generation of bovine primary ATII cells. These exhibit an ATII phenotype in 2D and 3D culture in our studies and are conducive to further study of the role of ATII cells in bovine respiratory diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

