Progression-free survival as a surrogate endpoint for overall survival in modern ovarian cancer trials: a meta-analysis
- PMID: 30093922
- PMCID: PMC6080081
- DOI: 10.1177/1758835918788500
Progression-free survival as a surrogate endpoint for overall survival in modern ovarian cancer trials: a meta-analysis
Abstract
Background: Progression-free survival (PFS) has been adopted as the primary endpoint in many randomized controlled trials, and can be determined much earlier than overall survival (OS). We investigated whether PFS is a good surrogate endpoint for OS in trials of first-line treatment for epithelial ovarian cancer (EOC), and whether this relationship has changed with the introduction of new treatment types.
Methods: In a meta-analysis, we identified summary data [hazard ratio (HR) and median time] from published randomized controlled trials. Linear regression was used to assess the association between treatment effects on PFS and OS overall, and for subgroups defined by treatment type, postprogression survival (PPS) and established prognostic factors.
Results: Correlation between HRs for PFS and OS, in 26 trials with 30 treatment comparisons comprising 24,870 patients, was modest (r2 = 0.52, weighted by trial sample size). The correlation diminished with recency: preplatinum/paclitaxel era, r2= 0.66; platinum/paclitaxel, r2= 0.44; triplet combinations, r2= 0.22; biologicals, r2= 0.30. The median PPS increased over time for the experimental (Ptrend = 0.03) and control arms (Ptrend = 0.003). The difference in median PPS between treatment arms strongly correlated with the difference in median OS (r2 = 0.83). In trials where the control therapy had median PPS of less than 18 months, correlation between PFS and OS was stronger (r2 = 0.64) than where the median PPS was longer (r2 = 0.48).
Conclusions: In EOC, correlation in the relative treatment effect between PFS and OS in first-line platinum-based chemotherapy randomized controlled trials is moderate and has weakened with increasing availability of effective salvage therapies.
Keywords: chemotherapy; clinical trials; ovarian cancer; therapy.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures



 predicted
linear relationship
predicted
linear relationship  ideal relationship.
ideal relationship.
 predicted
linear relationship
predicted
linear relationship  ideal relationship
◯ weights according to trial size.
ideal relationship
◯ weights according to trial size.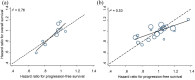
 predicted
linear relationship
predicted
linear relationship  ideal relationship
◯ weights according to trial size.
ideal relationship
◯ weights according to trial size.References
-
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64: 252–271. - PubMed
-
- Matulonis UA, Oza AM, Ho TW, et al. Intermediate clinical endpoints: a bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 2015; 121: 1737–1746. - PubMed
-
- Saad ED, Buyse M. Overall survival: patient outcome, therapeutic objective, clinical trial end point, or public health measure? J Clin Oncol 2012; 30: 1750–1754. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

