Recruitment & retention program for the NeuroNEXT SMA Biomarker Study: Super Babies for SMA!
- PMID: 30094386
- PMCID: PMC6072892
- DOI: 10.1016/j.conctc.2018.07.002
Recruitment & retention program for the NeuroNEXT SMA Biomarker Study: Super Babies for SMA!
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Contemp Clin Trials Commun. 2020 Dec 10;20:100688. doi: 10.1016/j.conctc.2020.100688. eCollection 2020 Dec. Contemp Clin Trials Commun. 2020. PMID: 33392412 Free PMC article.
Abstract
Background/aims: Recruitment and retention of research participants are challenging and critical components of successful clinical trials and natural history studies. Infants with spinal muscular atrophy (SMA) have been a particularly challenging population to study due to their fragile and complex medical issues, poor prognosis and, until 2016, a lack of effective therapies. Recruitment of healthy infants into clinical trials and natural history studies is also challenging and sometimes assumed to not be feasible.
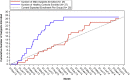
Methods: In 2011, our group initiated a two-year, longitudinal natural history study of infants with SMA and healthy infant controls to provide data to assist in the analysis and interpretation of planned clinical trials in infants with SMA. The recruitment goal was to enroll 27 infants less than 6 months of age with SMA and 27 age-matched healthy infants within the two-year enrollment period. A detailed recruitment and retention plan was developed for this purpose. In addition, a survey was administered to participant families to understand the determinants of participation in the study.
Results: All healthy infants were recruited within the study's first year and 26 SMA infants were recruited within the two-year recruitment period. Thirty-eight participant families responded to the recruitment determinants survey. Nearly half of respondents (18/38, 48%) reported that they first heard of the study from their physician or neurologist. The most common reason to decide to enroll their infant (22/38, 58%) and to remain in the study (28/38, 74%) was their understanding of the importance of the study. Thematic recruitment tools such as a study brochure, video on social media, and presentations at advocacy meetings were reported to positively influence the decision to enroll.
Conclusions: A proactive, thematic and inclusive recruitment and retention plan that effectively communicates the rationale of a clinical study and partners with patients, advocacy groups and the local communities can effectively recruit participants in vulnerable populations. Recommendations for the proactive integration of recruitment and retention plans into clinical trial protocol development are provided.
Keywords: Altruism; Healthy controls; Network; Social media; Spinal muscle atrophy.
Figures


References
-
- Bedlack R.S., Cudkowicz M.E. Clinical trials in progressive neurological diseases. Recruitment, enrollment, retention and compliance. Front. Neurol. Neurosci. 2009;25:144–151. - PubMed
-
- Comis R.L., Miller J.D., Aldige C.R., Krebs L., Stoval E. Public attitudes toward participation in cancer clinical trials. J. Clin. Oncol. 2003;21(5):830–835. - PubMed
-
- Sullivan-Bolyai S., Bova C., Deatrick J.A., Knafl K., Grey M., Leung K. Barriers and strategies for recruiting study participants in clinical settings. West. J. Nurs. Res. 2007;29(4):486–500. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

