Fluorescein Aqueous Angiography in Live Normal Human Eyes
- PMID: 30095604
- PMCID: PMC6218293
- DOI: 10.1097/IJG.0000000000001042
Fluorescein Aqueous Angiography in Live Normal Human Eyes
Abstract
Purpose: To evaluate aqueous humor outflow (AHO) in intact eyes of live human subjects during cataract surgery using fluorescein aqueous angiography.
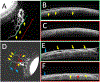
Methods: Aqueous angiography was performed in 8 live human subjects (56 to 86 y old; 2 men and 6 women). After anesthesia, fluorescein (2%) was introduced into the eye [either alone or after indocyanine green (ICG; 0.4%)] from a sterile, gravity-driven constant-pressure reservoir. Aqueous angiographic images were obtained with a Spectralis HRA+OCT and FLEX module (Heidelberg Engineering). Using the same device, anterior-segment optical coherence tomography (OCT) and infrared images were also concurrently taken with aqueous angiography.
Results: Fluorescein aqueous angiography in the live human eye showed segmental AHO patterns. Initial angiographic signal was seen on average by 14.0±3.0 seconds (mean±SE). Using multimodal imaging, angiographically positive signal colocalized with episcleral veins (infrared imaging) and intrascleral lumens (anterior-segment OCT). Sequential aqueous angiography with ICG followed by fluorescein showed similar segmental angiographic patterns.
Discussion: Fluorescein aqueous angiography in live humans was similar to that reported in nonhuman primates and to ICG aqueous angiography in live humans. As segmental patterns with sequential angiography using ICG followed by fluorescein were similar, these tracers can now be used sequentially, before and after trabecular outflow interventions, to assess their effects on AHO in live human subjects.
Figures


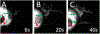



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

