The implementation of Xpert MTB/RIF assay for diagnosis of tuberculosis in Nepal: A mixed-methods analysis
- PMID: 30096174
- PMCID: PMC6086427
- DOI: 10.1371/journal.pone.0201731
The implementation of Xpert MTB/RIF assay for diagnosis of tuberculosis in Nepal: A mixed-methods analysis
Abstract
Background: Tuberculosis (TB) is a major public health problem in low and middle-income countries. Early detection and enrolment of TB cases is a challenge for National TB Programs.
Objective: To understand the performance and feasibility for scale-up of Xpert MTB/RIF assay for the TB diagnosis in Nepal.
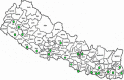
Design: Implementation research employed mixed-method sequential explanatory design. The results of Xpert MTB/RIF assay were analysed in 26 TB diagnostic centres where Xpert machines had been installed before 2015. In-depth interviews and focus group discussions were conducted with stakeholders, purposively selected to represent experiences in centres that were functioning well, poorly or not functioning.
Results: During a one-year period in 2015/16, 23,075 Xpert MTB/RIF assays were performed in 21 diagnostic centres with 22,288 people also tested by sputum microscopy. Among these, 77% had concordant (positive or negative) results, demonstrating fair agreement (Kappa score, 0.3) between test results. Test failure and positivity rates in diagnostic centres ranged from 2.6% to 13.4% and 6.5% to 49%, respectively. The number of cartridges per positive result varied from 2.3 to 10.2. Xpert assay was positive in 3314 (15% of all cases) sputum smear microscopy negative cases. Of 4280 bacteriologically confirmed cases by Xpert assay, 355 (8%) were rifampicin resistant. Xpert machines were no longer functioning regularly throughout the year in 5 diagnostic centres. The main barriers for effective implementation of Xpert in Nepal were the lack of: timely supply of cartridges; replacement of damaged modules; maintenance of Xpert machines; and stock verification for timely procurement of cartridges. Inadequate laboratory infrastructure for maintaining functional Xpert equipment further challenges implementation and scale-up.
Conclusion: The implementation of Xpert MTB/RIF assay has increased case-finding of TB and MDR-TB in Nepal. However, there is a need to improve laboratory performance and strengthen laboratory infrastructure for optimal utilisation and scale-up of Xpert.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Zumla A, Raviglione M, Hafner R, Fordham von Reyn C. Tuberculosis. N Engl J Med [Internet]. 2013. February 21 [cited 2016 Sep 24];368(8):745–55. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMra1200894 - DOI - PubMed
-
- WHO. Global Tuberculosis Report 2017, World Health Organization, Geneva 2017.
-
- WHO/SEA. Tuberculosis control in the South-East Asia Region / Annual Report 2016. 2016.
-
- NTC. Annual Report. Thimi, Bhaktapur; 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources