Phage therapy for respiratory infections
- PMID: 30096336
- PMCID: PMC6226339
- DOI: 10.1016/j.addr.2018.08.001
Phage therapy for respiratory infections
Abstract
A respiratory infection caused by antibiotic-resistant bacteria can be life-threatening. In recent years, there has been tremendous effort put towards therapeutic application of bacteriophages (phages) as an alternative or supplementary treatment option over conventional antibiotics. Phages are natural parasitic viruses of bacteria that can kill the bacterial host, including antibiotic-resistant bacteria. Inhaled phage therapy involves the development of stable phage formulations suitable for inhalation delivery followed by preclinical and clinical studies for assessment of efficacy, pharmacokinetics and safety. We presented an overview of recent advances in phage formulation for inhalation delivery and their efficacy in acute and chronic rodent respiratory infection models. We have reviewed and presented on the prospects of inhaled phage therapy as a complementary treatment option with current antibiotics and as a preventative means. Inhaled phage therapy has the potential to transform the prevention and treatment of bacterial respiratory infections, including those caused by antibiotic-resistant bacteria.
Keywords: Antibiotic-resistant bacteria; Bacteriophages (Phages); Biopharmaceutics; Formulation; Inhalation; Inhaled phage therapy; Respiratory infection.
Copyright © 2018. Published by Elsevier B.V.
Figures
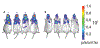
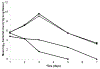
References
-
- Payne RJ, Jansen VA, Pharmacokinetic principles of bacteriophage therapy, Clin Pharmacokinet, 42 (2003) 315–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

