Developing Hollow-Channel Gold Nanoflowers as Trimodal Intracellular Nanoprobes
- PMID: 30096801
- PMCID: PMC6121537
- DOI: 10.3390/ijms19082327
Developing Hollow-Channel Gold Nanoflowers as Trimodal Intracellular Nanoprobes
Abstract
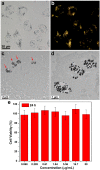
Gold nanoparticles-enabled intracellular surface-enhanced Raman spectroscopy (SERS) provides a sensitive and promising technique for single cell analysis. Compared with spherical gold nanoparticles, gold nanoflowers, i.e., flower-shaped gold nanostructures, can produce a stronger SERS signal. Current exploration of gold nanoflowers for intracellular SERS has been considerably limited by the difficulties in preparation, as well as background signal and cytotoxicity arising from the surfactant capping layer. Recently, we have developed a facile and surfactant-free method for fabricating hollow-channel gold nanoflowers (HAuNFs) with great single-particle SERS activity. In this paper, we investigate the cellular uptake and cytotoxicity of our HAuNFs using a RAW 264.7 macrophage cell line, and have observed effective cellular internalization and low cytotoxicity. We have further engineered our HAuNFs into SERS-active tags, and demonstrated the functionality of the obtained tags as trimodal nanoprobes for dark-field and fluorescence microscopy imaging, together with intracellular SERS.
Keywords: SERS; dark-field; fluorescence; gold nanoflowers; intracellular nanoprobes; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Vitol E.A., Orynbayeva Z., Friedman G., Gogotsi Y. Nanoprobes for intracellular and single cell surface-enhanced Raman spectroscopy (SERS) J. Raman Spectrosc. 2012;43:817–827. doi: 10.1002/jrs.3100. - DOI
-
- Kneipp K., Haka A.S., Kneipp H., Badizadegan K., Yoshizawa N., Boone C., Shafer-Peltier K.E., Motz J.T., Dasari R.R., Feld M.S. Surface-enhanced Raman Spectroscopy in single living cells using gold nanoparticles. Appl. Spectrosc. 2002;56:150–154. doi: 10.1366/0003702021954557. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

