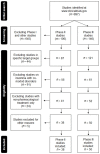
Inclusion and Exclusion Criteria of Clinical Trials for Insomnia
- PMID: 30096830
- PMCID: PMC6111373
- DOI: 10.3390/jcm7080206
Inclusion and Exclusion Criteria of Clinical Trials for Insomnia
Abstract
Randomized controlled trials (RCTs) have eligibility criteria for the inclusion of participants. Ideally, the RCT sample would be representative for the patient population that will use the drug under investigation. However, external validity may be at stake when applying too many or too restrictive eligibility criteria. The current two-part study examined (1) the currently applied eligibility criteria in Phase II and III RCTs examining sleep medication; (2) how these criteria match with the insomnia population as a whole; and (3) how inclusion rates can be changed by an adaptation of these criteria. In the first study, insomnia RCTs were screened at www.clinicaltrials.gov, and relevant eligibility criteria were identified. The second study comprised a survey among self-reported insomnia patients. It was determined to what extent RCT eligibility criteria match the characteristics of this patient population. Of the n = 519 patients that completed the survey only n = 2 (0.4%) met all eligibility criteria of current RCTs. RCT enrolment criteria are not representative for the insomnia patient population as a whole. Being less rigorous in applying upper or lower criteria limits results in a significant increase in the number of eligible patients, and increases the representativeness of RCTs for the insomnia patient population as a whole. The current analysis demonstrates that is important to thoroughly reconsider the use eligibility criteria and their inclusion ranges, and to have a theoretical basis for using them.
Keywords: clinical trial; efficacy; eligibility; exclusion criteria; inclusion criteria; insomnia; patient selection; recruitment; safety; screening.
Conflict of interest statement
Thomas Roth has received grants/research support from Aventis, Cephalon, Glaxo Smith Kline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth and Xenoport and has acted as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, Astra Zeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, Glaxo Smith Kline, Hypnion, Impax, Intec, Intra-Cellular, Jazz, Johnson & Johnson, King, Lundbeck, McNeil, Medici Nova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Procter-Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Joris Verster has received grants/research support from the Dutch Ministry of Infrastructure and the Environment, Janssen, Nutricia, Red Bull, Sequential, and Takeda, and has acted as a consultant for the Canadian Beverage Association, Centraal Bureau Drogisterijbedrijven, Clinilabs, Coleman Frost, Danone, Deenox, Eisai, Janssen, Jazz, Purdue, Red Bull, Sanofi-Aventis, Sen-Jam Pharmaceutical, Sepracor, Takeda, Transcept, Trimbos Institute, Vital Beverages, and ZBiotics. The other authors have no potential conflicts of interest to disclose.
Figures
References
-
- Heffron T.M. Insomnia Awareness Day Facts and Stats. Tratto Da Sleep Education. [(accessed on 1 April 2018)]; Available online: http://www.sleepeducation.org/news/2014/03/10/insomnia-awareness-day-fac....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous