Mesenchymal Stem Cell Migration during Bone Formation and Bone Diseases Therapy
- PMID: 30096908
- PMCID: PMC6121650
- DOI: 10.3390/ijms19082343
Mesenchymal Stem Cell Migration during Bone Formation and Bone Diseases Therapy
Abstract
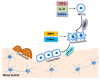
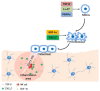
During bone modeling, remodeling, and bone fracture repair, mesenchymal stem cells (MSCs) differentiate into chondrocyte or osteoblast to comply bone formation and regeneration. As multipotent stem cells, MSCs were used to treat bone diseases during the past several decades. However, most of these implications just focused on promoting MSC differentiation. Furthermore, cell migration is also a key issue for bone formation and bone diseases treatment. Abnormal MSC migration could cause different kinds of bone diseases, including osteoporosis. Additionally, for bone disease treatment, the migration of endogenous or exogenous MSCs to bone injury sites is required. Recently, researchers have paid more and more attention to two critical points. One is how to apply MSC migration to bone disease therapy. The other is how to enhance MSC migration to improve the therapeutic efficacy of bone diseases. Some considerable outcomes showed that enhancing MSC migration might be a novel trick for reversing bone loss and other bone diseases, such as osteoporosis, fracture, and osteoarthritis (OA). Although plenty of challenges need to be conquered, application of endogenous and exogenous MSC migration and developing different strategies to improve therapeutic efficacy through enhancing MSC migration to target tissue might be the trend in the future for bone disease treatment.
Keywords: bone diseases; bone formation; mesenchymal stem cells; migration; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gemini-Piperni S., Takamori E.R., Sartoretto S.C., Paiva K.B.S., Granjeiro J.M., de Oliveira R.C., Zambuzzi W.F. Cellular behavior as a dynamic field for exploring bone bioengineering: A closer look at cell-biomaterial interface. Arch. Biochem. Biophys. 2014;561:88–98. doi: 10.1016/j.abb.2014.06.019. - DOI - PubMed
-
- Ozasa R., Matsugaki A., Isobe Y., Saku T., Yun H.S., Nakano T. Construction of human induced pluripotent stem cell-derived oriented bone matrix microstructure by using in vitro engineered anisotropic culture model. J. Biomed. Mater. Res. Part A. 2018;106:360–369. doi: 10.1002/jbm.a.36238. - DOI - PMC - PubMed
-
- Luu H.H., Song W.X., Luo X., Manning D., Luo J., Deng Z.L., Sharff K.A., Montag A.G., Haydon R.C., He T.C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2007;25:665–677. doi: 10.1002/jor.20359. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

