Biochemical Approaches for Understanding Iron-Sulfur Cluster Regeneration in Escherichia coli Lipoyl Synthase During Catalysis
- PMID: 30097094
- PMCID: PMC6501195
- DOI: 10.1016/bs.mie.2018.06.006
Biochemical Approaches for Understanding Iron-Sulfur Cluster Regeneration in Escherichia coli Lipoyl Synthase During Catalysis
Abstract
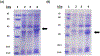
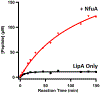
Lipoyl synthase (LipA in bacteria) is a radical S-adenosylmethionine (SAM) enzyme that catalyzes the second step of the de novo biosynthesis of the lipoyl cofactor: the insertion of sulfur at C6 and C8 of a pendant octanoyl chain. In addition to the [4Fe4S] cluster that is characteristic of the radical SAM (RS) enzymes, LipA contains a second [4Fe4S] cluster that, though controversial, has been proposed to be degraded during turnover to supply the inserted sulfur atoms. A consequence of this proposed role is that the destruction of its iron-sulfur cluster renders the enzyme in an inactive state. Recently, it was shown that Escherichia coli proteins NfuA or IscU can confer catalytic properties to E. coli LipA in vitro. In this chapter, we present methods for characterizing LipA and analyzing its activity in vitro, and provide strategies to monitor the pathway for the regeneration of LipA's auxiliary cluster by E. coli iron-sulfur carrier protein NfuA.
Keywords: Iron–sulfur cluster; Lipoic acid; Protein maturation; Radical; S-Adenosylmethionine.
© 2018 Elsevier Inc. All rights reserved.
Figures
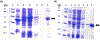
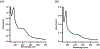
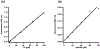
References
-
- Reed LJ, Busk BGD, Gunsalus IC, & Schnakenberg GHF (1951) Crystalline a-Lipoic Acid: A Catalytic Agent Associated with Pyruvate Dehydrogenase. Science 114:93. - PubMed
-
- Reed LJ, et al. (1953) Isolation, characterization and structure of α-lipoic acid. J. Am. Chem. Soc 75:1267–1270.
-
- Reed L (1974) Multienzyme complexes. Acc. Chem. Res 7:40–46.
-
- Miller JR, et al. (2000) Escherichia coli LipA is a lipoyl synthase: in vitro biosynthesis of lipoylated pyruvate dehydrogenase complex from octanoyl-acyl carrier protein. Biochemistry 39:15166–15178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

