Formate and Hydrogen as Electron Shuttles in Terminal Fermentations in an Oligotrophic Freshwater Lake Sediment
- PMID: 30097443
- PMCID: PMC6182907
- DOI: 10.1128/AEM.01572-18
Formate and Hydrogen as Electron Shuttles in Terminal Fermentations in an Oligotrophic Freshwater Lake Sediment
Abstract
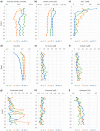
The energetic situation of terminal fermentations in methanogenesis was analyzed by pool size determinations in sediment cores taken in the oligotrophic Lake Constance, Germany. Distribution profiles of fermentation intermediates and products were measured at three different water depths (2, 10, and 80 m). Methane concentrations were constant below 10 cm of sediment depth. Within the methanogenic zone, concentrations of formate, acetate, propionate, and butyrate varied between 1 and 40 μM, and hydrogen was between 0.5 and 5 Pa. From the distribution profiles of the fermentation intermediates, Gibbs free energy changes for their interconversion were calculated. Pool sizes of formate and hydrogen were energetically nearly equivalent, with -5 ± 5 kJ per mol difference of free energy change (ΔG) for a hypothetical conversion of formate to hydrogen plus CO2 The ΔG values for conversion of fatty acids to methanogenic substrates and their further conversion to methane and CO2 were calculated with hydrogen and with formate as intermediates. Syntrophic propionate oxidation reached energetic equilibrium with formate as the sole electron carrier but was sufficiently exergonic if at least some of the electrons were transferred via hydrogen. The energetic consequences of formate versus hydrogen transfer in secondary and methanogenic fermentations indicate that both carrier systems are probably used simultaneously to optimize the energy yields for the partners involved.IMPORTANCE In the terminal steps of methane formation in freshwater lake sediments, fermenting bacteria cooperate syntrophically with methanogens and homoacetogens at minimum energy increments via interspecies electron transfer. The energy yields of the partner organisms in these cooperations have so far been calculated based mainly on in situ hydrogen partial pressures. In the present study, we also analyzed pools of formate as an alternative electron carrier in sediment cores of an oligotrophic lake. The formate and hydrogen pools appeared to be energetically nearly equivalent and are likely to be used simultaneously for interspecies electron transfer. Calculations of reaction energies of the partners involved suggest that propionate degradation may also proceed through the Smithella pathway, which converts propionate via butyrate and acetate to three acetate residues, thus circumventing one energetically difficult fatty acid oxidation step.
Keywords: energetics; fatty acids; methanogenesis; pool sizes; secondary fermentations; syntrophy.
Copyright © 2018 American Society for Microbiology.
Figures


References
-
- Bastviken D, Cole J, Pace M, Tranvik L. 2004. Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochem Cycles 18:GB4009.
-
- Bryant MP. 1979. Microbial methane production—theoretical aspects. J Animal Sci 48:193–201.
-
- Gujer W, Zehnder A. 1983. Conversion processes in anaerobic digestion. Water Sci Technol 15:127–167.
-
- Schink B, Stams AJM. 2013. Syntrophism among prokaryotes, p 471–493. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes—prokaryotic communities and ecophysiology. Springer-Verlag, Berlin, Germany.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

