Elevated ecto-5'-nucleotidase: a missing pathogenic factor and new therapeutic target for sickle cell disease
- PMID: 30097462
- PMCID: PMC6093737
- DOI: 10.1182/bloodadvances.2018015784
Elevated ecto-5'-nucleotidase: a missing pathogenic factor and new therapeutic target for sickle cell disease
Abstract
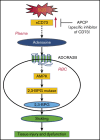
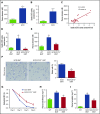
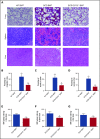
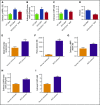
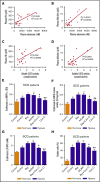
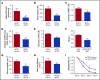
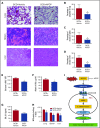
Although excessive plasma adenosine is detrimental in sickle cell disease (SCD), the molecular mechanism underlying elevated circulating adenosine remains unclear. Here we report that the activity of soluble CD73, an ectonucleotidase producing extracellular adenosine, was significantly elevated in a murine model of SCD and correlated with increased plasma adenosine. Mouse genetic studies demonstrated that CD73 activity contributes to excessive induction of plasma adenosine and thereby promotes sickling, hemolysis, multiorgan damage, and disease progression. Mechanistically, we showed that erythrocyte adenosine 5'-monophosphate-activated protein kinase (AMPK) was activated both in SCD patients and in the murine model of SCD. AMPK functions downstream of adenosine receptor ADORA2B signaling and contributes to sickling by regulating the production of erythrocyte 2,3-bisphosphoglycerate (2,3-BPG), a negative allosteric regulator of hemoglobin-O2 binding affinity. Preclinically, we reported that treatment of α,β-methylene adenosine 5'-diphosphate, a potent CD73 specific inhibitor, significantly decreased sickling, hemolysis, multiorgan damage, and disease progression in the murine model of SCD. Taken together, both human and mouse studies reveal a novel molecular mechanism contributing to the pathophysiology of SCD and identify potential therapeutic strategies to treat SCD.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Similar articles
-
Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia.Circulation. 2016 Aug 2;134(5):405-21. doi: 10.1161/CIRCULATIONAHA.116.021311. Circulation. 2016. PMID: 27482003 Free PMC article.
-
Elevated ecto-5'-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension.Circ Res. 2013 May 24;112(11):1466-78. doi: 10.1161/CIRCRESAHA.111.300166. Epub 2013 Apr 12. Circ Res. 2013. PMID: 23584256 Free PMC article.
-
Adenosine signaling in normal and sickle erythrocytes and beyond.Microbes Infect. 2012 Aug;14(10):863-73. doi: 10.1016/j.micinf.2012.05.005. Epub 2012 May 23. Microbes Infect. 2012. PMID: 22634345 Free PMC article. Review.
-
Elevated adenosine signaling via adenosine A2B receptor induces normal and sickle erythrocyte sphingosine kinase 1 activity.Blood. 2015 Mar 5;125(10):1643-52. doi: 10.1182/blood-2014-08-595751. Epub 2015 Jan 13. Blood. 2015. PMID: 25587035 Free PMC article.
-
Erythrocyte adaptive metabolic reprogramming under physiological and pathological hypoxia.Curr Opin Hematol. 2020 May;27(3):155-162. doi: 10.1097/MOH.0000000000000574. Curr Opin Hematol. 2020. PMID: 32141895 Free PMC article. Review.
Cited by
-
Survey of ribose ring pucker of signaling nucleosides and nucleotides.Nucleosides Nucleotides Nucleic Acids. 2020;39(1-3):322-341. doi: 10.1080/15257770.2019.1658115. Epub 2019 Aug 28. Nucleosides Nucleotides Nucleic Acids. 2020. PMID: 31460850 Free PMC article.
-
FT-4202, an oral PKR activator, has potent antisickling effects and improves RBC survival and Hb levels in SCA mice.Blood Adv. 2021 May 11;5(9):2385-2390. doi: 10.1182/bloodadvances.2020003604. Blood Adv. 2021. PMID: 33944896 Free PMC article.
-
Probiotic-Derived Ecto-5'-Nucleotidase Produces Anti-Inflammatory Adenosine Metabolites in Treg-Deficient Scurfy Mice.Probiotics Antimicrob Proteins. 2023 Aug;15(4):1001-1013. doi: 10.1007/s12602-023-10089-z. Epub 2023 May 13. Probiotics Antimicrob Proteins. 2023. PMID: 37178405 Free PMC article.
-
Generation and Export of Red Blood Cell ATP in Health and Disease.Front Physiol. 2021 Nov 5;12:754638. doi: 10.3389/fphys.2021.754638. eCollection 2021. Front Physiol. 2021. PMID: 34803737 Free PMC article. Review.
-
A2B adenosine receptor signaling and regulation.Purinergic Signal. 2025 Apr;21(2):201-220. doi: 10.1007/s11302-024-10025-y. Epub 2024 Jun 4. Purinergic Signal. 2025. PMID: 38833181 Free PMC article. Review.
References
-
- Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia a molecular disease. Science. 1949;110(2865):543-548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

